Biomarkers of the relationship of particulate matter exposure with the progression of chronic respiratory diseases
Article information
Abstract
A high level of particulate matter (PM) in air is correlated with the onset and development of chronic respiratory diseases. We conducted a systematic literature review, searching the MEDLINE, EMBASE, and Cochrane databases for studies of biomarkers of the effect of PM exposure on chronic respiratory diseases and the progression thereof. Thirty-eight articles on biomarkers of the progression of chronic respiratory diseases after exposure to PM were identified, four of which were eligible for review. Serum, sputum, urine, and exhaled breath condensate biomarkers of the effect of PM exposure on chronic obstructive pulmonary disease (COPD) and asthma had a variety of underlying mechanisms. We summarized the functions of biomarkers linked to COPD and asthma and their biological plausibility. We identified few biomarkers of PM exposure-related progression of chronic respiratory diseases. The included studies were restricted to those on biomarkers of the relationship of PM exposure with the progression of chronic respiratory diseases. The predictive power of biomarkers of the effect of PM exposure on chronic respiratory diseases varies according to the functions of the biomarkers.
INTRODUCTION
What is a biomarker?
According to the United States National Institutes of Health, a biomarker is a “characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes or pharmacologic responses to a therapeutic intervention” [1]. Thus, biomarkers are measurable indicators of molecular, histologic, radiographic, or physiologic characteristics [2].
Particulate matter (PM)
Air pollution is a major public health concern, and PM is a major component of polluted air [3]. PM comprises a combination of solid and liquid particles [4]. The typical chemical components of PM include sulfate, nitrate, and carbon in both elemental and organic forms, as well as organic substances, biological materials, and heavy metals [5]. An increase in PM exposure has been linked to the mortality and morbidity associated with respiratory diseases [3,6–8].
BIOMARKERS OF PM EXPOSURE: CHRONIC RESPIRATORY DISEASES
Chronic obstructive pulmonary disease (COPD)
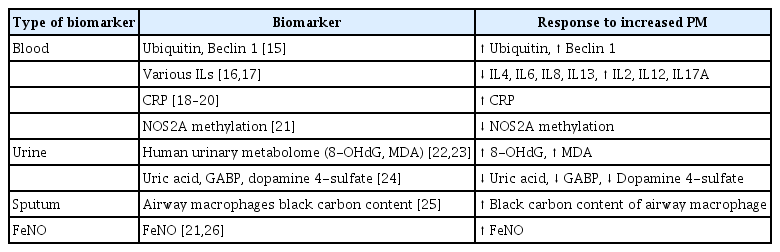
According to the Global Burden of Disease Study [9], exposure to fine PM (PM2.5, aerodynamic diameter ≤ 2.5 μm), which is linked to COPD, has increased, particularly in the elderly population [10]. Exposure to PM2.5 decreases lung function and aggravates respiratory diseases [11], leading to hospitalization, morbidity, and mortality [12–14]. Biomarkers are categorized as blood, urine, sputum, and fractional exhaled nitric oxide (FeNO) (Table 1).
Exposure to PM alters the levels of blood biomarkers in patients with COPD. Ubiquitin and beclin 1 levels change with increasing PM exposure [15]. The blood levels of some interleukins (ILs) increased, whereas the levels of others decreased, with increasing PM exposure [16,17]. An increased blood C-reactive protein (CRP) level is a marker of inflammation [18–20]. Also, biomarkers related to epigenetic regulation, such as reduced DNA methylation, have been identified [21].
Exposure to PM modulates the levels of urine biomarkers. The levels in urine of the metabolites 8-hydroxy-2′-deoxyguanosine (8-OHdG) and malondialdehyde (MDA) increased with increasing PM exposure [22,23]. Additionally, the levels of uric acid, glyceric acid 1,3-biphosphate (GABP), and dopamine-4-sulfate were elevated with increasing PM exposure [24].
Regarding sputum biomarkers, the black carbon (BC) content of airway macrophages (AMs), which target external environmental substances such as PM in the respiratory tract, increases as the PM level increases, suggesting potential as a biomarker of elevated PM exposure [25]. In addition, the level of FeNO, a marker of airway inflammation, increases after PM exposure in COPD [21,26].
Asthma
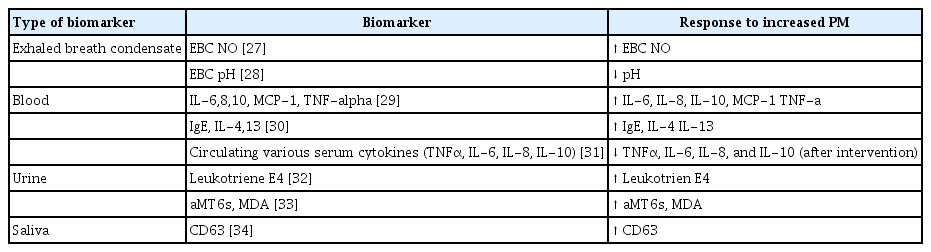
The air exhaled by patients with asthma contains biomarkers of PM exposure. The levels of biomarkers of asthma can be measured in exhaled breath condensate (EBC), blood, urine, and saliva (Table 2).
Whether exposure to PM is associated with oxidative stress was evaluated in an epidemiological study. The authors assessed exposure to PM by quantifying nitrite plus nitrate (NOx) in the EBC of 133 subjects with asthma or COPD [27]. The pH of EBC has promise as a biomarker of airway inflammation. In a cross-sectional study, asthma was significantly associated with a low EBC pH independent of indoor air quality [28].
Blood biomarkers have been suggested to be associated with PM exposure in patients with asthma. One study evaluated the effect of asthma status on the association between polluted air exposure and systemic effects by measuring blood levels of cytokines. Exposure to NO2 and PM was associated with higher levels of the proinflammatory cytokines IL-6 and TNF-α [29]. The levels of proinflammatory (IgE, IL-4, and IL-13) biomarkers were elevated following exposure to metals in PM2.5 in outdoor air around the homes of patients with asthma [30]. Indeed, air pollution, including PM, reduced the serum levels of TNF-α, IL-6, IL-8, and IL-10 in patients with asthma for 2 months [31].
Urinary leukotriene E4 is a biomarker of exposure to < 2.5 mm ambient polluted air (AMB-PM2.5) or secondhand smoke (SHS-PM2.5) [32]. In children with asthma, urinary 6-sulfatoxymelatonin (aMT6s), a surrogate marker of the circulating melatonin level, is associated with an increased level of MDA, a biomarker of systemic oxidative stress. Increased daily personal exposure to ozone (O3) and PM2.5 was associated with an increased level of aMT6s in patients with asthma [33].
Sputum markers of lung inflammation have been reported. Chemometric and regression results showed that the sputum CD63 level explained 72% of the variance in asthma incidence among patients exposed to PM2.5, and the models had high predictive accuracies. CD63 is associated with the activation and degranulation of eosinophils and neutrophils [34].
BIOMARKERS OF THE EFFECT OF PM EXPOSURE ON THE PROGRESSION OF CHRONIC RESPIRATORY DISEASES
Most biomarkers of the effect of PM exposure on chronic respiratory diseases have been evaluated in a small number of epidemiological cross-sectional studies. This literature review focuses on the mechanisms underlying the progression of chronic respiratory diseases linked to PM, with the aim of identifying biomarkers of the effect of PM exposure in patients with chronic respiratory diseases.
Methods
Eligibility criteria
Disease progression is defined in terms of incidence, progression, or the clinical deterioration of patients with chronic respiratory diseases (e.g., COPD, asthma, or idiopathic pulmonary fibrosis [IPF]). However, none of the studies focused on IPF; therefore, we reviewed studies on COPD and asthma. We performed a literature search based on the following inclusion criteria: population: patients with chronic respiratory diseases (COPD and asthma); intervention and comparator: PM (PM2.5, PM10) exposure; outcomes: biomarkers of disease progression according to PM exposure; published after 2013; and full-text articles in English. The exclusion criteria were as follows: studies that did not target patients; studies that did not involve exposure to PM; studies that did not report the outcomes of interest; and duplicate studies.
Information sources and search strategy
We searched the Ovid MEDLINE, Ovid EMBASE, and Cochrane Central Register of Controlled Trials electronic databases on 20 January 2023, for articles describing studies of biomarkers of the effect of PM exposure on chronic respiratory diseases published after 1 January 2013. The search was performed using the following combinations of keywords: (“particulate matter” OR “PM10” OR “PM2.5”) AND (“chronic obstructive pulmonary disease” OR “COPD”) AND (“biomarker” OR “Biomarkers”) AND (“Incidence” OR “Disease progression” OR “Clinical deterioration”). Chronic respiratory disease was considered to encompass diseases such as asthma and IPF. Synonyms for PM, biomarkers, chronic respiratory disease, and disease progression were included among Medical Subject Headings (MeSH) terms, EMBASE subject headings, and text words. The search was limited to studies published in the English language. The search strategy is shown in Supplementary File 1.
Study selection and statistical analysis
Two expert pulmonologists (JK and SJC) screened the titles and abstracts of articles according to the inclusion criteria. Each author independently assessed the eligibility of the studies; conflicts were resolved by discussion. Full texts were assessed by two authors (JK and SJC) to reach a final decision on article inclusion or exclusion. Disagreement was resolved by discussion with a third author (WJK). No other statistical analysis was performed.
Risk of bias assessment
A validated tool was used to evaluate risk of bias based on study design. The Risk of Bias Assessment Tool for Non-randomized Studies (RoBANS) 2.0 was used for nonrandomized studies [35]. This tool comprises eight domains: possibility of target group comparisons, target group selection, confounders, exposure measurement, blinding of assessors, outcome assessment, incomplete outcome data, and selective outcomes. Each domain was classified as low, high, or unclear risk of bias. Quality assessments were conducted by two authors (JK and SJC), and disagreements were resolved by discussion with a third author (WJK).
Results
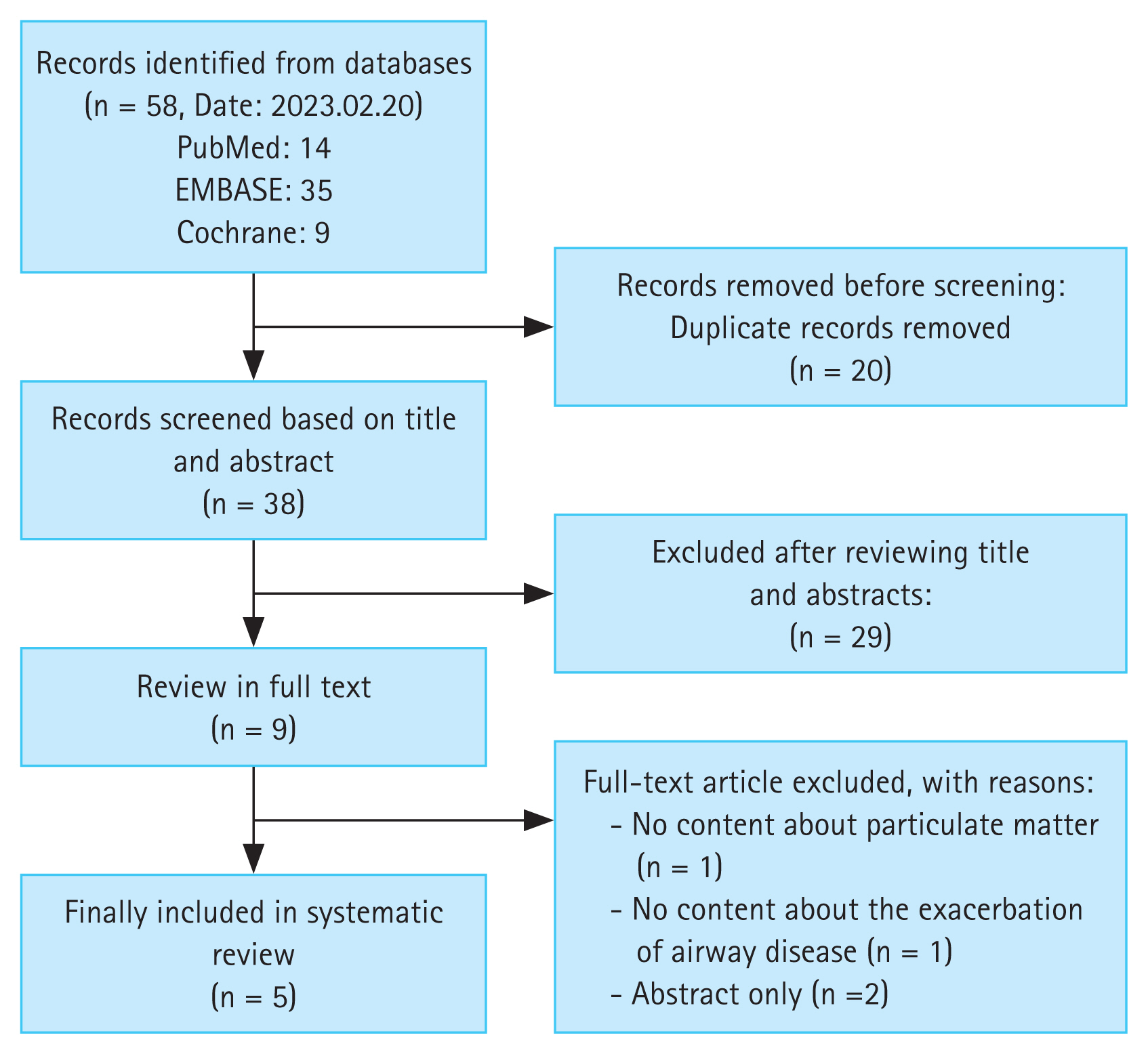
A total of 58 studies were identified, and 20 duplicate studies were removed before screening. Of the remaining 38 studies, 30 were excluded after screening the titles and abstracts. Subsequently, the full texts of eight studies were reviewed for eligibility. Ultimately, four studies were included (Fig. 1). Two articles contained related contents by same authors. The excluded studies and reasons for their exclusion are provided in Supplementary File 2.
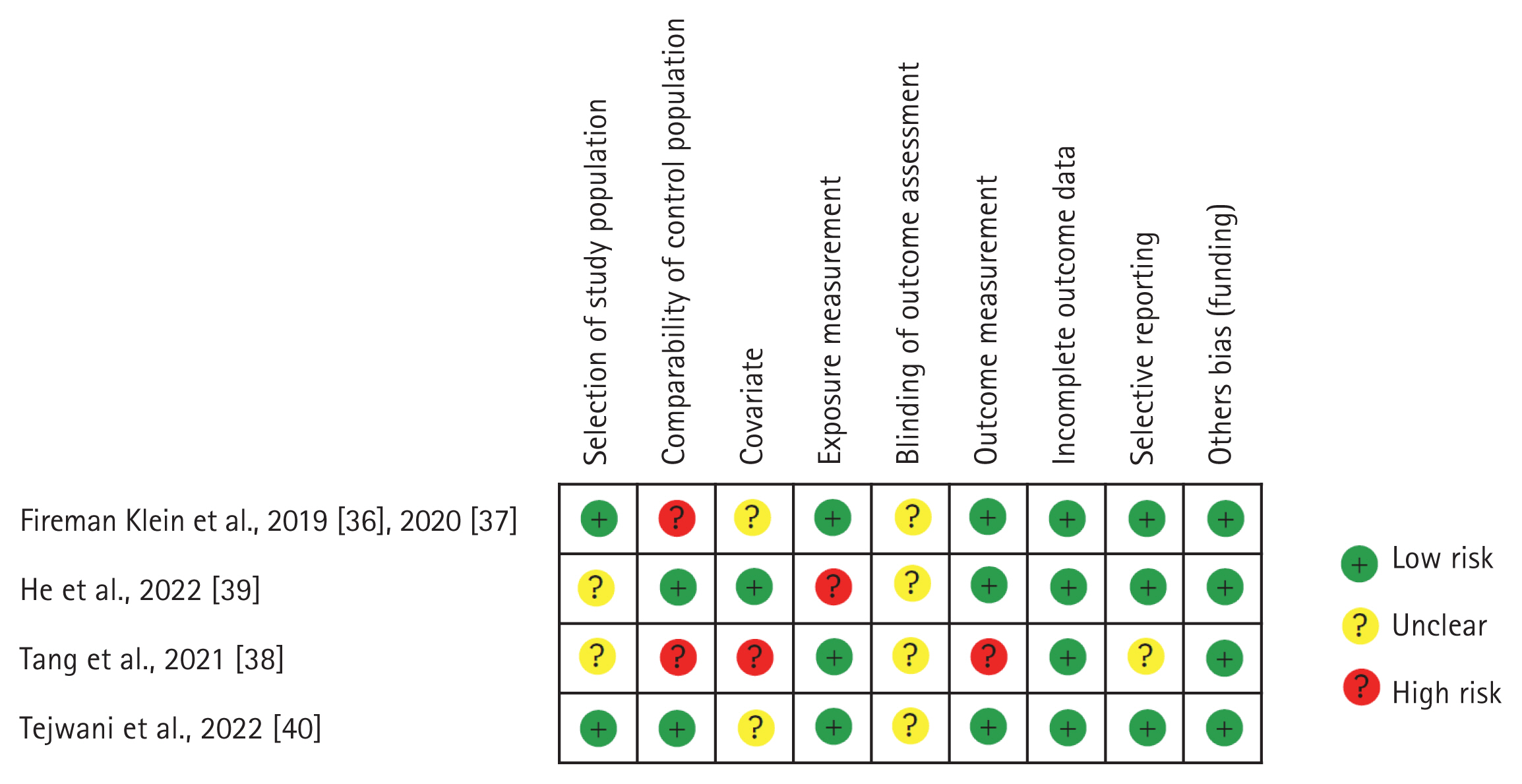
The characteristics of the included studies are listed in Table 3. Three studies [36–38] had a high risk of bias in the possibility of target group comparisons domain, and another [38] in the confounders domain. One study [39] had a high risk of bias in the exposure measurement domain, and another [38] in the outcome domain (Fig. 2).
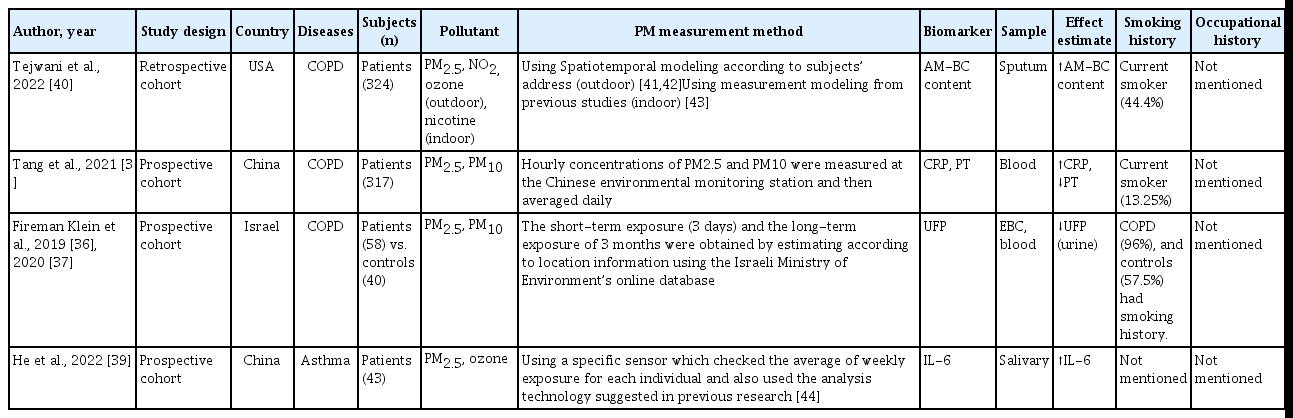
Characteristics of the included studies on the relationships of biomarkers of PM exposure with the progression of chronic respiratory diseases
Few studies have evaluated the relationships of biomarkers of PM exposure with the progression of chronic respiratory diseases. This review focuses on biomarkers of the effect of PM on chronic respiratory diseases (COPD and asthma) and their biological plausibility.
BC in AMs
AMs, which are important immune cells for tissue repair in COPD, are exposed to airborne pollutants, including PM. AM-BC, measured as the mean BC cross-sectional area (mm2) per macrophage, is a biomarker of exposure to airborne PM and tobacco smoke. Tejwani et al. [40] investigated the relationships of the AM-BC level and PM2.5 exposure with sputum, spirometry, and respiratory outcomes. They used spatiotemoral modeling according to subjects’ address for estimating outdoor pollution [41,42] and also used mesurement modeling from previous study for estimating indoor polution [43]. The results showed that the AM-BC level has potential as a biomarker of COPD exacerbation and PM exposure [40]. An increase in the indoor PM2.5 concentration was associated with an increase in AM-BC area (β = 9.96%, 95% confidence interval [CI] 0.47–20.4%) and percentage (β = 13.1%, 95% CI 3.49–23.6%). An increase in AM-BC area was not associated with an increase in overall COPD exacerbation frequency over 1 year but was associated with an increased risk of severe exacerbations (odds ratio [OR] = 1.52, 95% CI 1.01–2.28). Similarly, an increase in AM-BC percentage was not correlated with an increase in the overall exacerbation frequency but was associated with an increased risk of severe exacerbations (OR = 2.19, 95% CI 1.0–44.63).
CRP and prothrombin time (PT)
Abnormal inflammatory responses to environmental factors, including PM, have been linked to COPD. CRP, a marker of the systemic inflammatory response, has been proposed as a biomarker of COPD. Tang et al. measured the blood CRP level and PT in 317 patients with acute COPD exacerbation (AECOPD) 1 day before admission; the CRP level was increased during COPD exacerbation and PM elevation [38]. Of the subjects, 13.25% were smokers, and their occupational histories were not evaluated. Patients with AECOPD exposed to > 25 mg/L PM2.5 had a significantly shorter PT and a significantly higher CRP level compared to those exposed to ≤ 25 mg/L PM2.5.
Ultrafine particles (UFPs)
UFPs are small, toxic materials that reflect the severity of inflammation in patients with COPD. Einat et al. evaluated the UFP levels in the EBC and blood of patients with COPD and 40 healthy controls. A low UFP level in EBC and a high level in serum were indicative of high PM exposure [36]. In addition, a low UFP level in EBC was an independent predictor of frequent exacerbations (OR = 3.6, 95% CI 1.06–7.97; p = 0.04). Therefore, a low UFP level in EBC has potential as a biomarker of COPD exacerbation and high PM exposure [37].
Salivary IL-6 level
Exposure to air pollution causes inflammation in the oral cavity, which may exacerbate airway inflammation and asthma symptoms. He et al. [39] reported that asthmatic children had an elevated salivary IL-6 level, a biomarker of an increased PM2.5 level, and exacerbation of asthma symptoms. They used a specific sensor which checked the average of weekly exposure for each individual and also used the analysis technology suggested in previous research [44]. Additionally, the asthma control test (ACT) score decreased as the salivary IL-6 level increased, indicating worsening of asthma symptoms.
Epidemiologic perspectives
Environmental factors, including PM exposure, have a marked effect on the development and course of chronic respiratory diseases, including asthma and COPD [7,8]. Therefore, monitoring and reducing PM exposure is important for controlling these diseases [1]. Biomarkers are important considerations when formulating regulations to restrict emissions [45]. Therefore, the identification of biomarkers of PM exposure would improve the management of chronic respiratory diseases.
In this study, we described biomarkers of the effect of PM exposure on asthma and COPD and reviewed studies on biomarkers of PM exposure during exacerbations of those diseases. Only one study evaluated biomarkers associated with PM exposure in patients with COPD [46].
This review had several limitations. Most of the included studies evaluated a single disease-related biomarker in populations distinguished based on age, race, country, and respiratory disease status compared to healthy controls or the effect of PM exposure on the levels of biomarkers. This introduces a risk of selection bias when attempting to generalize the results (Fig. 2). Additionally, although standardized assessment methods facilitate the assessment of PM exposure, uncertainty remains because of rapid technological advancements. Other potential covariates—such as components of outdoor/indoor air pollutants, smoking history, and occupational history—which are risk factors for disease, should also be evaluated. These covariates were not accounted for in some studies, so further research is needed.
CONCLUSIONS
Few studies have explored the relationships of PM biomarkers with the progression of chronic respiratory diseases. The predictive power of biomarkers of the effect of PM exposure on chronic respiratory diseases varies according to the functions of the biomarkers. Because of the limitations of the included studies, there are few data on biomarkers of the effect of PM exposure on chronic respiratory diseases. Further prospective studies are required to establish regulations to reduce airborne PM and develop public health strategies for the prevention and management of respiratory diseases caused by environmental factors.
Acknowledgments
This research was supported by the Korea Environment Industry and Technology Institute through the Core Technology Development Project for Environmental Disease Prevention and Management, funded by the Korea Ministry of Environment (number 2022003310009).
Notes
CRedit authorship contributions
Junghyun Kim: conceptualization, methodology, data curation, writing - original draft, writing - review & editing; Soo Jie chung: methodology, writing - original draft; Woo Jin Kim: conceptualization, methodology, writing - review & editing, funding acquisition
Conflicts of interest
The authors disclose no conflicts.
Funding
This study was supported by the Korea Environment Industry and Technology Institute through the Core Technology Development Project for Environmental Disease Prevention and Management, funded by the Korea Ministry of the Environment (number 2022003310009).