Efficacy and safety of denosumab treatment for Korean patients with Stage 3b–4 chronic kidney disease and osteoporosis
Article information
Abstract
Background/Aims
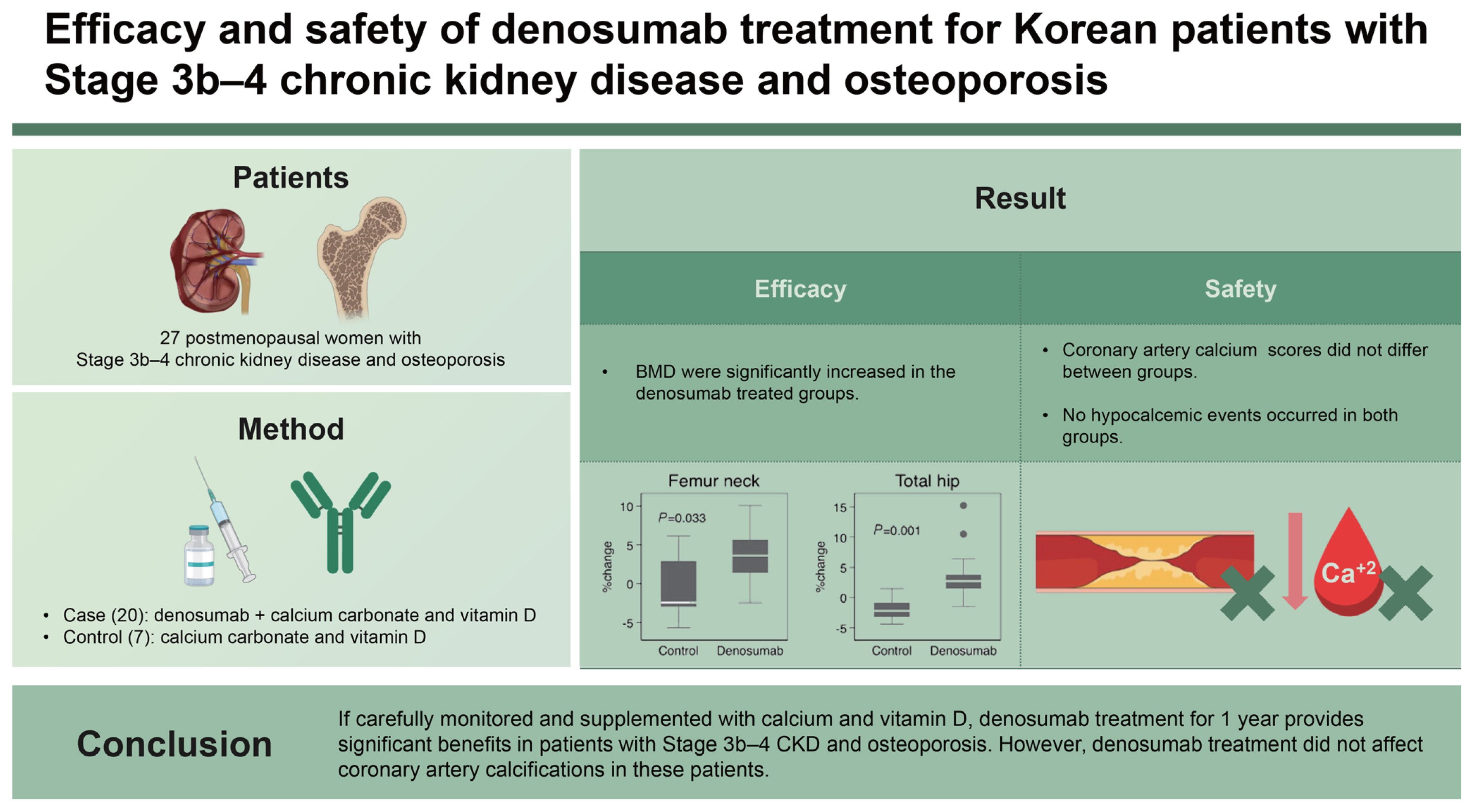
We evaluated the efficacy and safety of denosumab treatment in severe chronic kidney disease (CKD) patients with osteoporosis. We also investigated whether the treatment affects the coronary artery calcifications.
Methods
Twenty-seven postmenopausal women with Stage 3b–4 CKD and osteoporosis were enrolled. Twenty patients received denosumab plus calcium carbonate and vitamin D, and seven controls received calcium carbonate and vitamin D for 1 year. Dual-energy X-ray absorptiometry and coronary artery calcium (CAC) scoring computed tomography were performed before and after treatment. Hypocalcemic symptoms and serum calcium levels were evaluated.
Results
After 1 year of treatment, the percent changes of femur neck (3.6 ± 3.2% vs. −0.7 ± 4.4%, p = 0.033) and total hip (3.4 ± 3.8% vs. −1.9 ± 2.1%, p = 0.001) bone mineral density (BMD) were significantly increased in the denosumab treated group compared to the control group. However, the percent change of lumbar spine BMD did not differ between two groups (5.6 ± 5.9% vs. 2.7 ± 3.9%, p = 0.273). The percent change of bone alkaline phosphatase was significantly different in the denosumab-treated group and control group (−31.1 ± 30.0% vs. 0.5 ± 32.0%, p = 0.027). CAC scores did not differ between groups. No hypocalcemic events occurred in both groups.
Conclusions
If carefully monitored and supplemented with calcium and vitamin D, denosumab treatment for 1 year provides significant benefits in patients with Stage 3b–4 CKD and osteoporosis. However, denosumab treatment did not affect coronary artery calcifications in these patients.
INTRODUCTION
The incidence rate of osteoporotic fractures in patients with chronic kidney disease (CKD) are 2–17 times higher and associated with higher morbidity and mortality rates than that in the general population [1,2]. Renal osteodystrophy is a complex disorder of the bone that includes mineral imbalance, osteitis fibrosa cystica caused by secondary hyperparathyroidism, adynamic bone disease, and osteoporosis [3]. It is also considered a systemic disease that includes extra-bone complications, such as vascular and soft tissue calcification and the so-called CKD-mineral and bone disorder (CKD-MBD) [3]. Owing to such a complex pathophysiology, it is difficult to accurately diagnose osteoporosis in patients with CKD without bone biopsy, and until recently, osteoporosis in patients with CKD is often neglected and untreated. Although it serves as the gold standard test for the differential diagnosis of osteitis fibrosa and adynamic bone disease, bone biopsy is remarkably limited in routine clinical practice.
However, the recent change in the Kidney Disease: Improving Global Outcomes (KDIGO) treatment guidelines for CKD-MBD has led to a decrease in the tendency to use calcium (Ca) as a phosphate binder, and the KDIGO has updated the guidelines to recommend fracture risk assessment in patients with CKD by dual-energy X-ray absorptiometry (DXA) since 2017 [3]. In patients with stage 1–4 CKD, osteoporosis is the main cause of fracture in most cases, unless disorders in Ca, phosphorus (P), and parathyroid hormone (PTH) levels are severe, and the bone mineral density (BMD) test using DXA is a significantly useful tool for evaluating fracture risk in these patients [2]. In addition, with the advent of denosumab, interest in the treatment of osteoporosis in patients with renal insufficiency has increased. Accordingly, the rate of diagnosis of osteoporosis in patients with CKD is increasing; however, there are insufficient data about the long-term effects and safety outcomes of denosumab in these patients, especially those with severe renal impairment. Denosumab is a monoclonal antibody with an affinity for receptor activator of nuclear factor κB ligand (RANKL), prevents osteoclast formation by binding to RANKL and blocking the interaction between RANKL and RANK (receptor on the osteoclast surface), and reduces bone resorption in osteoporosis [4]. Denosumab is a Food and Drug Administration-approved drug for the treatment of osteoporosis and can be used in patients with impaired renal function because it is not renally excreted, unlike bisphosphonates [5]. Phase III trials of denosumab in the treatment of osteoporosis have suggested that denosumab treatment may be used both effectively and safely in stages 1–3 mild to moderate CKD [6]. However, the side effect of hypocalcemia can occur frequently and severely in patients with severe renal insufficiency, and it is more problematic in real-world practice because there are several cases where it is used inadvertently without monitoring the Ca level during treatment [7,8].
In previous large-scale prospective studies, the coronary artery calcium (CAC) score has strongly predicted cardiovascular (CV) events in addition to traditional CV risk factors [9]. Coronary artery calcification progresses during the course of CKD from early stage and was found to be in close association with MBDs [10]. In particular, the prevalence of vascular calcification and CV events increases markedly when the estimated glomerular filtration rate (eGFR) is < 45 mL/min [11,12]. Compelling evidences from previous human epidemiological literature have suggested that the osteoprotegerin/RANK/RANKL system is a common link between bone disease and CV outcomes, especially atherosclerosis, arterial calcification, and CV disease [13–15].
Therefore, this study aimed to determine the efficacy and side effects of denosumab in patients with CKD stage 3b–4 (eGFR between 15 and 44 mL/min) when it is used with sufficient doses of oral Ca and vitamin D and is closely monitored. In this study, osteoporosis was diagnosed using DXA-BMD. Bone turnover marker changes and Ca, P, and PTH levels were monitored. In addition, the effects of denosumab on vascular and extraskeletal calcifications and CV risk were evaluated using CAC score and serum homocysteine level changes.
METHODS
Trial design and participants
This was a pilot clinical study prospectively conducted at Nowon Eulji University Hospital from June 3, 2020, to January 20, 2023. Postmenopausal women aged > 50 years with Stage 3b–4 CKD (eGFR between 15 and 44 mL/min) and diagnosed with osteoporosis by BMD T-score ≤ −2.5 (mean values over at least two parts of the lumbar spine, femur neck, or total hip) were enrolled. Baseline Ca, and P levels were within the reference range in this study. GFR was calculated using the creatinine equation (CKD-EPI 2021), which is as follows: eGFRcr = 142 × min(Scr/κ, 1)α × max(Scr/κ, 1)−1.200 × 0.9938Age × 1.012 (if female), where Scr is the standardized serum creatinine in mg/dL (κ = 0.7 [female] or 0.9 [male], α = −0.241 [female] or −0.302 [male]), min(Scr/κ, 1) is the minimum of Scr/κ or 1.0, and max(Scr/κ, 1) is the maximum of Scr/κ or 1.0 [16]. The exclusion criteria were osteoporotic patients with one of the following conditions; endocrine diseases such as Cushing’s syndrome, thyrotoxicosis or hypogonadism, rheumatoid arthritis, drugs affecting bone metabolism including corticosteroid, severe hyperparathyroidism (intact parathyroid hormone [iPTH] > 500 pg/mL), uncontrolled hyperphosphatemia, previous osteoporosis treatment within 3 years, previous vertebral fracture history on spine X-ray and dialysis treatment.
After initial screening, participants were randomly assigned to receive 60 mg of denosumab subcutaneously every 6 months for 1 year or to the control group in a 3:1 rato. All participants were instructed to take daily Ca carbonate 1,000–1,250 mg (elemental Ca 400–500 mg) and vitamin D 800–1,000 IU. BMDs, bone turnover markers, homocysteine levels, and CAC scores were measured at baseline and after 1 year of treatment. Total Ca, ionized Ca, and iPTH levels were measured at every visits (at baseline, at 2 weeks, thereafter at 3, 6, and 12 months). We also evaluated hypocalcemic symptoms.
Measurements
Baseline clinical data, including age, sex, smoking history, cause of CKD, comorbidities, relevant medication history, and fracture history were collected. Height and weight were measured. Body mass index (BMI) was calculated as weight (in kilograms) divided by the square of height (in meters). Blood samples were drawn after overnight fasting (> 12 h). Ca, P, 25-hydroxyvitamin D (25(OH)D), iPTH, bone alkaline phosphatase (ALP), C-terminal cross-linked telopeptide of type I collagen (CTX), and hosmocysteine levels were measured at baseline and follow-up in all patients. We measured blood chemistry by an enzymatic technique using an ADVIA 2400 analyzer (Siemens Healthineers, Erlangen, Germany). Serum 25(OH)D levels, bone ALP levels, and CTX levels were measured using the Liaison 25(OH) vitamin D Total test (DiaSorin-LIAISON® XL; DiaSorin S.p.A, Vercelli, Italy), ADIVA 1650 Chemistry system (Bayer Diagnostics, Leverkusen, Germany), electrochemiluminescence immunoassay (Roche, Indianapolis, IN, USA), respectively. Homocysteine and iPTH levels were measured using an ADVIA Centaur XP Immunoassay System ( Siemens Healthineers) and chemiluminescent immunoassay (Siemens Healthineers). Lumbar spine and hip BMDs were measured using DXA (Lunar Prodigy; GE Lunar Corp., Madison, WI, USA). The spine BMD values were calculated as the average of values measured in L1–L4 (mean values over at least two parts of the lumbar spine) excluding vertebral changes due to structural problems, including collapse, degenerative disease, surgery, or internal artifacts. Vertebrae were also excluded from the analysis if the T-score of a vertebra was greater than 1 standard deviation (SD) of an adjacent vertebra. Osteoporosis was defined as the lowest T-score of ≤ −2.5 [9]. The coefficient of variation of the BMD measurements was ≤ 3.0%. All patients underwent imaging of coronary arteries using a 64-slice multi-detector CT scanner (Siemens SOMATOM Definition AST; Siemens Healthineers). Methodologies for the acquisition and interpretation of scans have been previously published [10]. The presence of coronary artery calcification was identified and analyzed by software (SINGVIA, Siemens Healthineers) and CAC score was calculated as described Agatston et al. [11] and Pletcher et al. [12]. Calcification within the coronary arteries is recognized if it has a density greater than 130 Hounsfield unit (HU) and covers an area larger than 1 mm2. The Agatston score is determined by taking the area of each calcified section and multiplying it by a weighted computed tomography number, which is scored between 1–4 depending on the maximum HU of that specific section. CAC scores were separately obtained for each of the main epicardial coronary arteries (left main artery, left anterior descending artery, left circumflex artery, and right coronary artery). The total Agatston score is the cumulative score of each lesion found in the coronary arteries. All the scans were read by a single expert investigator, blinded for the subjects’ clinical profile.
Statistical analyses
All continuous variables are expressed as means ± SDs. Comparisons between groups were performed using the Mann–Whitney U test and chi-squared test. To examine the changes in BMD, bone turnover marker changes, and CAC scores, the values at baseline and at 12 months were compared. The changes in parameters were used in analyses as percentage changes, calculated using the following formula: (values at 12 months – values at baseline)/values at baseline × 100 (%). The Mann–Whitney U test was used for nonparametric analysis. Pearson’s correlation test was used to evaluate the correlations among BMD changes, baseline bone turnover markers, and 25(OH)D levels. All p values for differences of serial changes between denosumab-treated and control group were calculated by panel analysis with random effect models. A p value of < 0.05 indicated statistical significance. All statistical analyses were performed using STATA 16.1 (StataCorp, College Station, TX, USA).
Ethics statement
The Institutional Review Board (IRB) of Nowon Eulji University Hospital approved the study protocol (IRB approval number Eulji 2020-04-012, Clinical Research information Service, cris.nih.go.kr number 20230208-006). This study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All participants provided written informed consent prior to participation.
RESULTS
Baseline characteristics according to treatment
A total of 27 female patients with CKD Stage 3b–4 and osteoporosis were included. All participants were of Korean ethnicity. The analysis of this study corresponds to a per protocol analysis. One patient in the denosumab treatment arm who was lost to follow-up was not included in the analysis. Table 1 shows the baseline characteristics of the study population according to treatment. Within the Denosumab group, there were 11 patients diagnosed with CKD stage 3b and 9 patients in stage 4. In comparison, the control group consisted of 4 patients in stage 3b and 3 patients in stage 4. None of the participants were current smokers, ex-smokers, or drinkers. No significant differences were found in medication history; demographics; serum creatinine levels; eGFRs; BMIs; lumbar and hip BMDs; bone turnover markers; 25(OH)D, iPTH, and homocysteine levels; and CAC scores between the two groups. However, the prevalence of diabetes was higher in the control group than in the denosumab-treated group (85.7 vs. 30.0%, p = 0.011).
Primary outcomes
Bone mineral density and bone turnover marker changes according to denosumab treatment
There were more significant improvements in BMD in the denosumab-treated group compared with the control group (Fig. 1A). After 1 year, the percent changes of femur neck (3.6 ± 3.2% vs. −0.7 ± 4.4%, p = 0.033) and total hip BMDs (3.4 ± 3.8% vs. −1.9 ± 2.1%, p = 0.001) from baseline were significantly increased in the denosumab treated groups compared to the control group. However, the percent change of lumbar spine BMD did not differ between two groups (5.6 ± 5.9% vs. 2.7 ± 3.9%, p = 0.273).

The figure shows median changes in bone mineral density (A), bone turnover markers (B), coronary calcium scores, and homocysteine levels (C) according to the treatment groups. Error bar means interquartile range. ALP, alkaline phosphatase; CTX, C-terminal cross-linked telopeptide of type I collagen; PTH, parathyroid hormone.
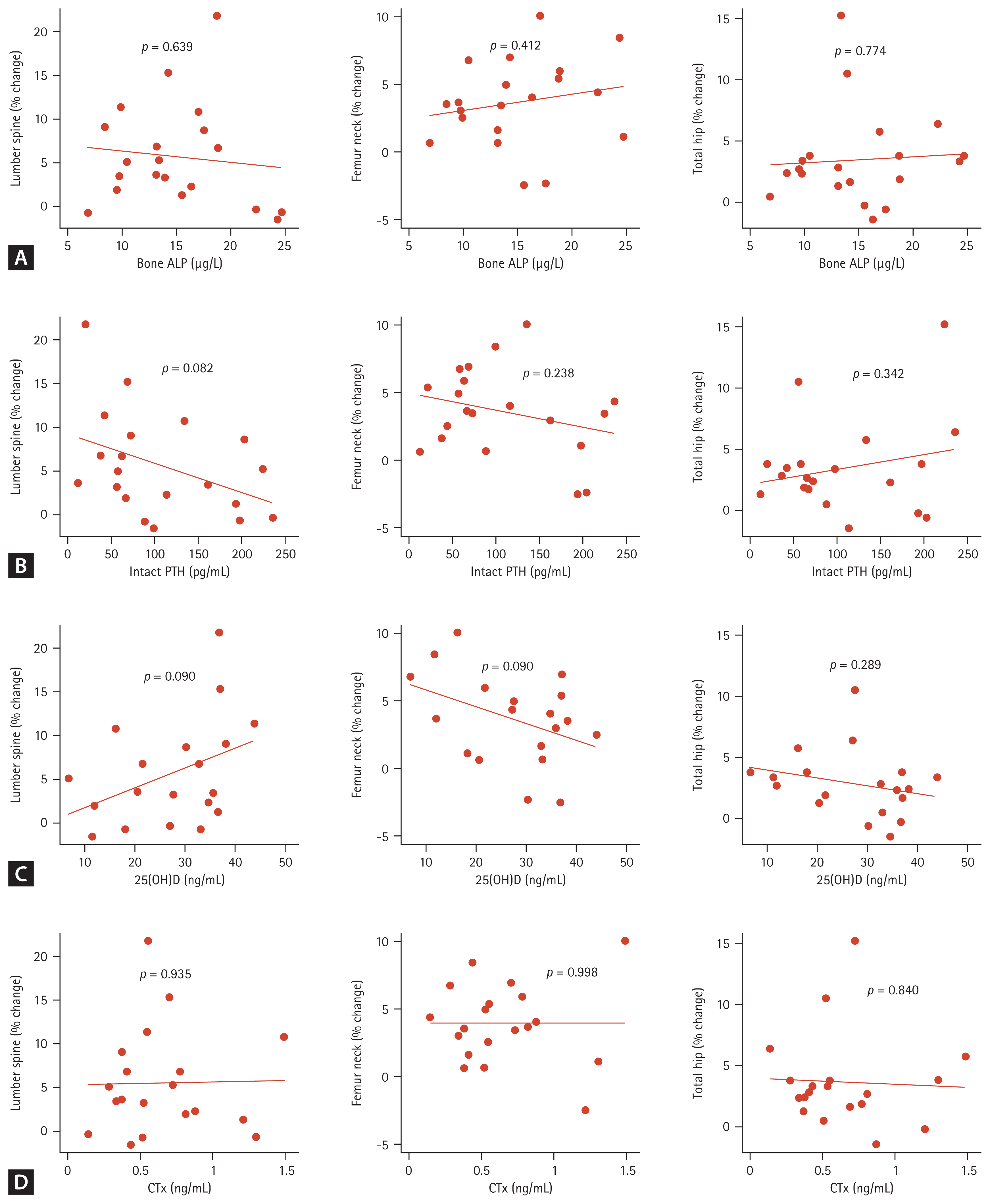
Among the bone turnover markers, the bone ALP percent changes from baseline were significantly different in the denosumab-treated and control groups (−31.1 ± 30.0% vs. 0.5 ± 32.0%, p = 0.027) (Fig. 1B). The CTX percent changes were also significantly different in two groups (−49.2 ± 29.9% vs. −17.7 ± 28.0%, p = 0.025) (Fig. 1B). In the denosumab-treated group, correlations among BMD changes, baseline bone turnover marker, and 25(OH)D levels were analyzed, but no significant correlation was found (Fig. 2). In the denosumab administered group, there was a significant increase in 25(OH)D levels from 27.9 ± 10.8 ng/mL to 33.5 ± 8.7 ng/mL after a year (p = 0.021 by Wilcoxon signed-rank test). In the control group, it increased from 23.7 ± 16.5 ng/mL to 35.8 ± 15.5 ng/mL, but it was not statistically significant (p = 0.079 by Wilcoxon signed-rank test).
Calcium, phosphorus, and parathyroid hormone level changes according to denosumab treatment
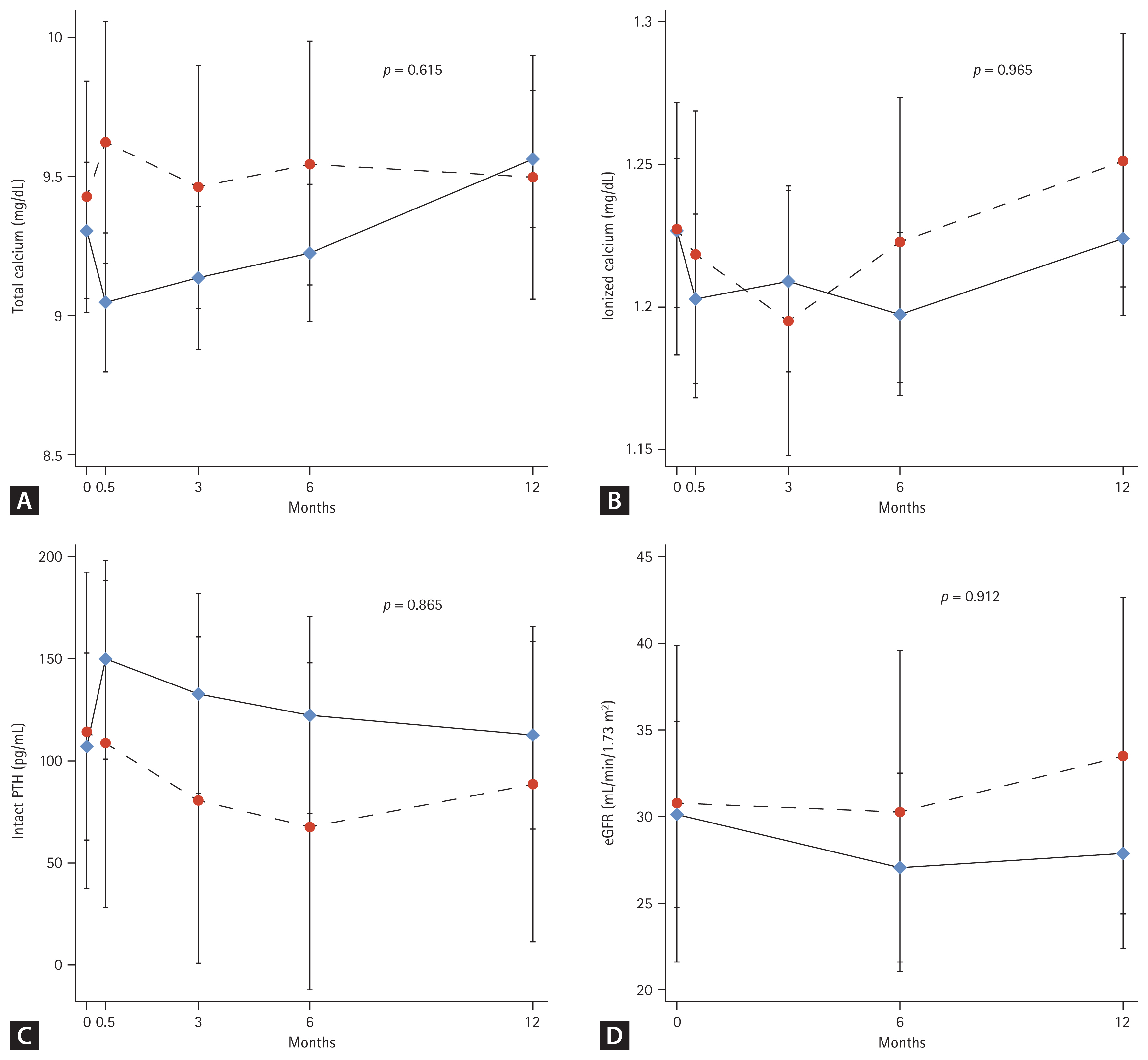
Total Ca, ionized Ca, and iPTH levels were measured at every visits (baseline and 2 weeks, thereafter at 3, 6, and 12 months). No significant changes from baseline levels or between-group differences were observed in total Ca (Fig. 3A), ionized Ca (Fig. 3B), and iPTH (Fig. 3C) levels throughout the study period. The eGFR changes were not different between the groups during the treatment period (Fig. 3D).
Adverse events during study period
It is well known that denosumab-induced hypocalcemia commonly occurs within the first few weeks of treatment in CKD, so we measured calcium level at baseline, at 2 weeks, thereafter at 3, 6 and 12 months. We also evaluated hypocalcemic symptoms at every visits. However, we could not find any symptomatic or laboratory hypocalcemia during the treatment period among the participants. No osteoporotic fractures occurred in the denosumab-treated or control groups during the study period. None of the enrolled patients progressed to end-stage renal disease (ESRD) stage requiring dialysis treatment during the study period. During the one-year administration period, we did not observe any confirmed side effects like osteonecrosis of the jaw, atypical femur fracture or urinary stones.
Secondary outcomes
Coronary artery calcium score changes according to denosumab treatment
When the change in the total CAC score after 1 year, expressed as the Agatston score, was compared according to denosumab treatment, changes of 21.1 ± 54.3% and 13.7 ± 34.1% in the denosumab-treated and control groups were observed, and there was no significant difference between the two groups (p = 0.563) (Fig. 1C). There was no significant decrease in homocysteine levels with 1 year of denosumab treatment.
DISCUSSION
In this study, we demonstrated that the use of denosumab for 1 year in patients with advanced CKD was effective in improving BMD. No osteoporotic fractures occurred during the study period among the participants. In addition, denosumab showed no side effects and good compliance, suggesting the potential use of denosumab as a promising therapeutic agent in this disease group. Femur neck and total hip BMDs were significantly increased from baseline after 1 year in the denosumab-treated group compared to the control group, who received Ca carbonate (elemental Ca 400–500 mg) and vitamin D only. Different from previous studies, with sufficient replacement of Ca and vitamin D and close monitoring, there were no symptomatic and laboratory hypocalcemic episodes in this study. In the case of spine BMD, no significant % change difference was found between the denosumab-treated group and the control group, as 2.7% increase in spine BMD was also confirmed in the control group with administration of Ca and vitamin D. The level of change of 5.6% increase in spine BMD in denosumab group is similar in percentage change to what is observed in other treatment study using denosumab in hemodialysis [13]. In the FREEDOM trial, a major clinical trial on denosumab, postmenopausal women with osteoporosis were treated with denosumab. After 1 year of treatment, lumbar spine BMD increased by an average of about 5.2% in the denosumab group [4]. In the subgroup analysis of the FREEDOM trial, patients with CKD stage 3a and 3b showed a rise in spinal BMD of 21.7 and 23.7%, respectively, after three years of treatment with denosumab [6]. Previous studies have demonstrated that total hip BMD is the best predictor of future fracture and that CKD has a greater effect on hip BMD deterioration than spine BMD [14,15]. The finding that denosumab was effective in improving femur neck and total hip BMDs in our study has important implications in this sense.
Moreover, the use of vitamin D, instead of activated vitamin D, had a sufficient preventive effect on hypocalcemia at this CKD Stage. There were no significant changes in Ca, ionized Ca, P, or iPTH levels throughout the study. In previous small randomized controlled trials and observational off-label studies conducted in patients with CKD, denosumab-induced hypocalcemia was observed commonly to a severe degree in ESRD [13,17,18]. Moreover, low baseline Ca and 25(OH)D levels, higher tartrate-resistant acid phosphatase 5b (TRAP5b) levels, absence of Ca carbonate replacement, low bone turnover, and high bone turnover disease are predictors of hypocalcemia [13,17,18].
Among the bone turnover markers, bone ALP level significantly decreased after treatment, and this study confirmed once again that it is a useful marker for predicting the therapeutic effect of osteoporosis treatment in patients with CKD [19]. The ALP change of about −31.1 ± 30% over a year in our study is a similar rate of change to a previous study, even though percentage change in bone ALP can vary between studies and populations [13]. There are limitations in interpreting bone turnover markers in CKD because several of these markers and their metabolites are excreted by the kidneys, and procollagen type I N-terminal propeptide and CTX are representative of these markers [20]. For this reason, in our study, we could confirm a significant decrease in CTX in the denosumab group, but caution is required in interpretation. In contrast, bone ALP, a bone formation marker, is unaffected by renal function and is associated with fracture rate and mortality in patients with CKD [19,21]. In addition, bone ALP, when interpreted in combination with iPTH, correlates well with the histomorphometry of bone biopsy in assessing adynamic bone disease [20]. Recently, TRAP5b, which is unaffected by renal dysfunction, has been a good bone resorption marker in CKD [22]. Therefore, further studies using this new marker is required to understand the exact pathophysiology.
Another hypothesis of this study is that denosumab may affect vascular and extraskeletal calcification by modulating RANKL activity in predialysis CKD. Several studies have suggested that RANK/RANKL signaling plays a role in vascular calcification by promoting osteogenic differentiation of vascular smooth muscle cells (VSMCs), which are the primary cell type involved in vascular calcification. RANKL can induce VSMCs to differentiate into osteoblast-like cells and deposit calcium in the extracellular matrix, leading to vascular calcification. Additionally, RANKL may activate inflammatory pathways and promote the recruitment of immune cells to the vessel wall, which can further contribute to vascular calcification [23,24].
However, in this study, the secondary outcome CAC scores did not change after denosumab administration. The study observation period of 1 year may have been too short to observe the effect. Another probable cause for this lack of effect is that the degree of vascular calcification is less severe, less progressive, and difficult to change in predialysis patients than in dialysis patients. According to a study on hemodialysis patients previously conducted in Japan, denosumab caused a 25% regression of aortic calcification during 8 years of treatment, but this effect was not confirmed in the first year of administration [25]. In the same period, aortic calcification progressed by 57% in the control group [25]. In the subgroup analysis of the FREEDOM study, 36-month administration of denosumab among patients with high CV risk did not result in a significant change in the aortic calcification score evaluated by lateral spine X-ray, and the result did not differ when analyzed by baseline GFR [26]. Although the duration of the study was shorter, we enrolled patients with more advanced CKD compared with the FREEDOM study. Additional studies with longer follow-up durations are required to reach a definitive conclusion.
In this study, changes in homocysteine levels were investigated as a risk factor for CV disease, osteoporosis and fracture, but no significant decrease in homocysteine levels was found with 1 year of denosumab treatment. It is well-known that homocysteine appears to be an independent risk factor for CV diseases in both general populations and patients with CKD. The mechanisms for increased homocysteine levels in CKD are believed to be due to decreased renal excretion and impaired renal metabolism [27]. Previous studies suggested that elevated levels of homocysteine may be also a risk factor for osteoporosis and fracture in older individuals [28]. The exact mechanisms underlying this relationship are not fully understood, but may involve a negative effect of homocysteine on BMD and bone turnover [28–30]. To our knowledge, there are no studies investigating the effect of denosumab on homocysteine levels in humans.
This study has some limitations. First, this was a single-center study with a small sample size and relatively short duration of treatment. Second, although we used useful clinical bone markers instead of bone biopsies, we might have included patients with other metabolic bone disease associated with CKD rather than osteoporosis. Third, as fractures did not occur in either group, additional long-term follow-up studies are required to confirm whether BMD improvement leads to fracture prevention. Throughout the thesis, it should be clarified that this is a pilot study that mainly focus on changes in BMD, bone turnover markers, and coronary calcium scoring over the course of one year, and that osteoporotic fractures are not the main outcomes. Fourth, there may be criticism that osteoporosis treatment was not performed in the control group, but in this study, bone density was measured and calcium and vitamin D were administered to the control group according to the KDIGO guidelines.
Despite these limitations, to the best of our knowledge, this is the first prospective clinical study in Korean patients with Stage 3b–4 CKD and osteoporosis to demonstrate that denosumab can be used safely and effectively when supplemented with adequate Ca and vitamin D. Denosumab, when carefully used according to each individual’s bone turnover and Ca and phosphate balance, provided significant benefits in patients with Stage 3b–4 CKD with no side effects. However, the study observed the treatment effect over a span of one year and did not present evidence supporting its continuation beyond that period. Furthermore, there are no concensus about appropriate medication to switch after denosumab treatment in CKD patients. Additionally, there has not been a study to date investigating the impact of denosumab treatment on bone histomorphometry in CKD patients. Therefore, further research is needed to clarify the effect on pathophysiology and safety.
As a secondary outcome, a year-long denosumab treatment did not significantly reduce the CAC scores in these patients. Moreover, denosumab did not demonstrate any effect on the homocysteine level, which is an indirect marker of CV disease in CKD. Further nationwide researches are needed to fully understand the potential effects of denosumab on CV health.
KEY MESSAGE
1. In Korean patients with Stage 3b–4 CKD and osteoporosis, 1 year treatment with denosumab improved femur neck and total hip BMDs.
2. There were no symptomatic and laboratory hypocalcemic episodes with sufficient calcium and vitamin D supplementations.
3. One year of denosumab treatment was ineffective in reducing the CAC scores or homocysteine levels in these patients.
Notes
CRedit authorship contributions
Jin Taek Kim: conceptualization, investigation, formal analysis, writing - original draft, writing - review & editing; You Mi Kim: methodology, investigation, data curation, formal analysis, writing - original draft, writing - review & editing; Kyong Yeun Jung: conceptualization, methodology, resources, investigation, data curation, writing - original draft, writing - review & editing; Hoonsung Choi: investigation, data curation, formal analysis, writing - original draft, writing - review & editing; So Young Lee: conceptualization, methodology, investigation, data curation, formal analysis, writing - original draft, writing - review & editing, funding acquisition; Hyo-Jeong Kim: conceptualization, methodology, investigation, data curation, formal analysis, writing - original draft, writing - review & editing, funding acquisition
Conflicts of interest
The authors disclose no conflicts.
Funding
This study was funded by DALIM BIOTECH.