Endoscopic mucosal resection using anchored snare Tip-in versus precut technique for small rectal neuroendocrine tumors
Article information
Abstract
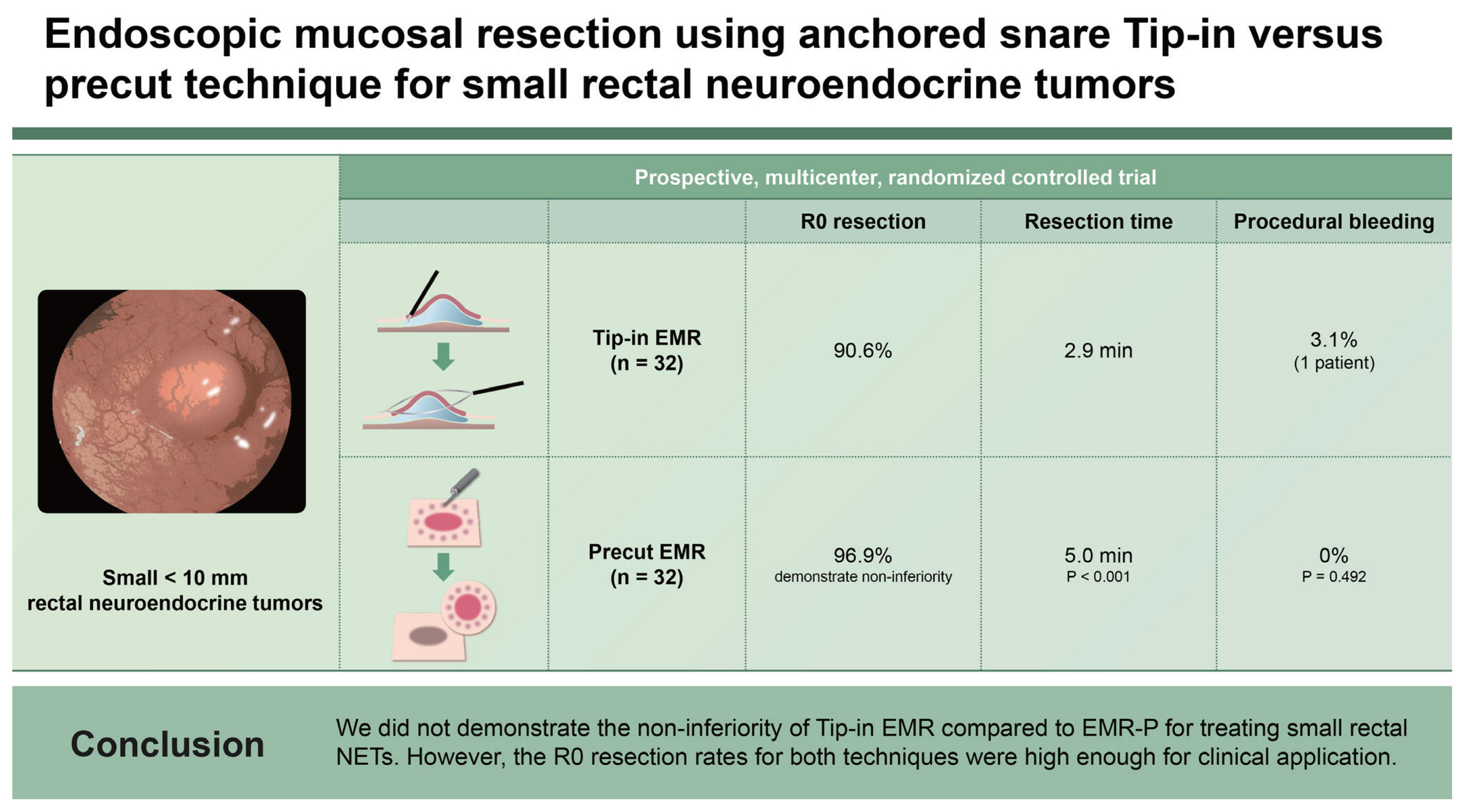
Background/Aims
Small rectal neuroendocrine tumors (NETs) can be treated with modified endoscopic mucosal resection (EMR). However, an optimal EMR method remains to be established. We aimed to assess the non-inferiority of Tip-in EMR versus precut EMR (EMR-P) for treating rectal NETs.
Methods
This prospective, multicenter, randomized controlled trial enrolled patients with rectal NETs of < 10 mm in diameter. The patients were randomly assigned to EMR-P and Tip-in EMR groups in a 1:1 ratio. Primary outcome was margin-negative (R0) resection rate between the two methods, with a noninferiority margin of 10%.
Results
Seventy-five NETs in 73 patients, including 64 eligible lesions (32 lesions in each, EMR-P and Tip-in EMR groups), were evaluated. In a modified intention-to-treat analysis, R0 resection rates of the EMR-P and Tip-in EMR groups were 96.9% and 90.6%, respectively, which did not demonstrate non-inferiority (risk difference, −6.3 [95% confidence interval: −18.0 to 5.5]). Resection time in the EMR-P group was longer than that in the Tip-in EMR group (p < 0.001). One case of intraprocedural bleeding was reported in each group.
Conclusions
We did not demonstrate the non-inferiority of Tip-in EMR compared to EMR-P for treating small rectal NETs. However, the R0 resection rates for both techniques were high enough for clinical application.
INTRODUCTION
Rectal neuroendocrine tumors (NETs) are subepithelial tumors that arise from the enteroendocrine cells located in the lower and middle third of the rectal crypt [1]. The incidence of rectal NETs has increased following the introduction of the classification by the World Health Organization, widespread use of colonoscopy and increased awareness among clinicians [2–6]. Rectal NETs < 10 mm are considered suitable for endoscopic treatment due to their very low risk of metastases [7]. Most rectal NETs invade the submucosa, even if they are small in size [8]; therefore, a deep submucosal layer should be included in the resected specimen to achieve a histologically complete resection. Conventional endoscopic mucosal resection (EMR) is less likely to achieve a histologically complete resection than the modified EMR or endoscopic submucosal dissection (ESD) [9]. The latter is an effective method for achieving a histologically complete resection of small rectal NETs; however, it is technically difficult for an average endoscopist [10].
Among modified EMR methods, cap-assisted EMR (EMR-C) or EMR with band ligation (EMR-L) requires addition of specialized devices once a rectal NET is established or suspected [11–14]. This implies that the scope must be withdrawn from the colorectum in order to attach the devices dedicated to each procedure. Therefore, EMR-C and EMR-L are inconvenient for the removal of an incidentally detected rectal NET. Precut endoscopic mucosal resection (EMR-P) is a modified EMR technique that creates a circumferential mucosal incision using the tip of a snare before snaring the target lesion [15]. Additionally, EMR-P is relatively easy to perform and does not require dedicated devices. A histologically complete resection rate using EMR-P for small rectal NETs ranges between 81.2–96.7% [15–19]. However, a 6.3% perforation rate was reported in a study [17], and EMR-P is considered an independent risk factor for perforation after endoscopic resection of laterally spreading colorectal tumors [20]. Thus, EMR-P may still be challenging for average endoscopists who are skillful at conventional EMR but not ESD.
Conversely, anchored snare-tip EMR or ‘Tip-in’ EMR is a modified technique that anchors the snare-tip into a mucosal slit to prevent slipping of the snare while capturing a lesion [21,22]. According to previous studies, Tip-in EMR achieves higher complete resection rates of colorectal neoplasia compared to that of conventional EMR [23–26]. A retrospective study showed that the histological complete resection rates of Tip-in EMR and EMR-C for small rectal NETs were 94.1 and 88.2%, respectively (p = 0.673) [27]. Thus, the Tip-in EMR may be attempted before performing EMR-P for treating rectal NET. We hypothesized that Tip-in EMR is not inferior to EMR-P in removing small rectal NETs. Therefore, in this study, we aimed to assess the non-inferiority of Tip-in EMR compared to EMP-P in the therapeutic outcomes of small rectal NETs.
METHODS
Study design and patient enrollment
This multicenter, prospective randomized controlled trial (RCT) included five medical institutions in Korea. Patients with suspected or established rectal NETs who were referred for endoscopic resection at each center were screened. Inclusion criteria were as follows: patients with established rectal NETs or lesions showing endoscopic findings compatible with rectal NETs; and those aged 20–79 years. Exclusion criteria were as follows: lesion size ≥ 10 mm; lesions referred for rescue treatment after incomplete endoscopic resection; lesions showing a non-lifting sign; and patients with uncontrolled coagulopathy. This study was approved by the Institutional Review Board (IRB) of each institution, and written informed consent was obtained from all the participants (IRB No. and approval date are shown in Supplementary Table 1). This RCT was registered at the Clinical Research Information Service (CRIS), Republic of Korea (registration identifier, KCT0005148), and enrollment began following the CRIS registration.
The study participants were randomly assigned to EMR-P and Tip-in EMR groups in a 1:1 ratio before the procedure. A blocked randomization method was used to allocate the same number of patients to each treatment group. An independent study coordinator notified each endoscopist of the randomization results before the procedure. We collected laboratory and clinical data regarding underlying diseases and medications of the participants.
Endoscopic procedure
All the endoscopic procedures were performed by five endoscopists, each with an experience of at least 200 cases of EMR-P. One endoscopist (D.H.Y.) had experience in Tipin EMR for > 50 cases of rectal NETs before planning this study, and he shared the basic principle and techniques of Tip-in EMR for rectal NETs with the other endoscopists in an offline educational meeting [27]. Before enrolling the patients, the other four endoscopists had performed Tip-in EMR for > 10 rectal NETs.
A high-definition colonoscope (CF-HQ 290 EVIS LUCERA ELITE; Olympus Corporation, Tokyo, Japan) with a transparent cap and 13-mm oval type stiff snare with a 0.42-mm wire diameter (Captivator; Boston Scientific, Marlborough, MA, USA) were used for both the methods. The EMR-P procedure was performed as follows: a mixture of normal saline, 1:100,000 epinephrine and indigo carmine were injected submucosally to lift the lesion. Using the tip of the snare, a fully circumferential incision was made around the lesion. Next, the lesion was snared along the circumferential incision and resected with an electric current (Fig. 1). For the Tip-in EMR procedures, the same submucosal solution was injected around the lesion, and a mucosal slit of 2–5 mm in width was made approximately 5 mm from the proximal edge of the lesion [27]. Next, the tip of the snare was anchored into the mucosal incision, and the snare was slowly opened. Furthermore, the snare was gently pressed towards the anchor site to maintain the width of the snare and ensure a leverage effect for the lesion [27]. The snare sheath was pressed downward to entrap the deep submucosal tissue together with the lesion [27]. Finally, the captured lesion was resected using the electric current (Fig. 2).
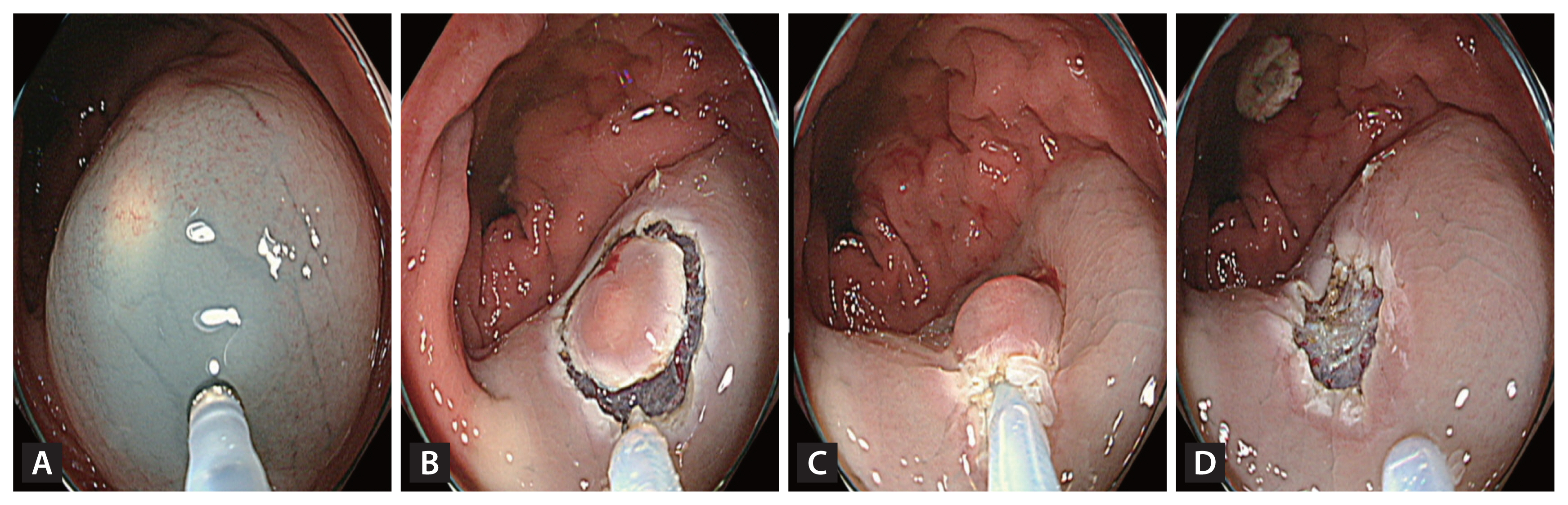
Images of precut endoscopic mucosal resection. (A) Inject submucosal solution around the lesion. (B) Use the snare tip to precut circumferentially around the lesion with an adequate margin. (C) Capture the lesion including the margin using the snare. (D) Image after resection.

Images of Tip-in endoscopic mucosal resection (A) After injecting a submucosal solution, create a slit using the snare tip. (B) Fix the snare tip onto the slit and expand the snare to capture the lesion extensively. (C) Close the snare with an adequate margin. (D) Image after resection.
Procedure time was measured by an assistant using a stopwatch mounted on the endoscopic imaging system. In case of intraprocedural bleeding, hemoclips or electrocoagulation using the snare tip was performed for hemostasis. Clipping, electrocauterization, or prophylactic approximation of mural defects were allowed to prevent delayed adverse events and performed based on the endoscopists’ decision.
Histological outcomes
Resected specimens were evaluated by board-certified pathologists in each center. The longest diameter of the NET, mitosis count, Ki-67 proliferation index, depth of invasion, and resection margin statuses were reported according to a standardized protocol provided by a central pathologist (S.M.H.). Safety resection margin was also measured, which is defined as the closest distance between a tumor and normal tissue during an en bloc resection.
Definition and study outcomes
An margin-negative (R0) resection was defined as an en bloc resection with histologically tumor-free resection margins. The resection time was defined as the time from the first needle injection of submucosal solution to the gross complete resection of the target lesion. Post-procedure processing time was defined as the time required for exploring the resection site and performing additional endoscopic procedures, including hemostatic treatment for immediate bleeding, prophylactic clipping or electrocauterization of exposed vessels, and endoscopic closure of suspected/established perforation. Total procedure time was the sum of resection and postprocedure processing time.
All the patients visited outpatient clinics at each center between 2–4 weeks after the endoscopic treatment. Reported adverse events included intraprocedural bleeding, immediate/delayed postprocedural bleeding, perforation, and postpolypectomy coagulation syndrome. The definition of each adverse event is shown in Supplementary Table 2.
Primary outcome was the difference in R0 resection rate between the endoscopic resection methods. Secondary outcomes included en bloc resection rate, procedure time, and adverse events.
Sample size calculation and statistical analysis
In a previous study, R0 resection rate for small rectal NETs using Tip-in EMR was 94.1% [27], whereas that of EMR-P varied between 69.1–93.1% [15,17,18]. We extracted the number of patients from previous studies who underwent EMR-P with their corresponding R0 resection rates (81.4%) and conducted a pooled analysis. Based on these results, our estimated R0 resection rates were 81.4 and 94.1% for the EMR-P and Tip-in EMR techniques, respectively. Therefore, we hypothesized that Tip-in EMR is not inferior to EMR-P regarding R0 resection rate for small rectal NETs, with a non-inferiority margin of 10%. To verify the non-inferiority between the two groups with a statistical power of 80% and significance level of 2.5%, we calculated a sample size of 32 for each group. Assuming a 10% attrition rate for the study, a minimum of 35 patients was required in each group.
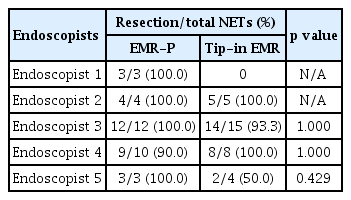
Continuous variables were presented as medians and interquartile ranges (IQRs) or mean and standard deviation (SD), while categorical variables were presented as absolute numbers and percentages. Differences in patient characteristics were analyzed using chi-square or Fisher’s exact tests for categorical variables and t-tests for continuous variables. All the analyses were performed on a modified intention-to-treat basis; patients who were histologically not diagnosed with NET were excluded from the analysis. We compared the R0 resection rates of the two methods by the endoscopist to see if there was a difference among the operators. A two-sided p value of < 0.05 was considered statistically significant. All the analyses were performed using R software (version 4.0.2; R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Study participants
Between November 2020 and July 2021, 75 NETs in 73 patients were included in the study. The patients were randomly assigned to the EMR-P and Tip-in EMR groups at a ratio of 1:1. In four cases, EMR was converted to an ESD technique, according to the endoscopist’s decision without any attempt at a modified EMR technique. In one case, no visible lesion was present. Six lesions were identified as non-NET lesions after the endoscopic resection (no remnant NET, 4; chronic colitis, 2). Overall, 32 NETs were included in each of the EMR-P and Tip-in EMR groups (Fig. 3). Baseline characteristics of each group are presented in Table 1. The median sizes of the tumors estimated during the endoscopic procedures were 4.5 mm (IQR, 2.0–9.0) and 6.0 mm (IQR, 3.0–10.0) in the EMR-P and Tip-in EMR groups, respectively. Only one case had an ulcer or depression on the surface.

Flow chart of enrollment and randomization of the study participants. EMR-P, precut endoscopic mucosal resection; ESD, endoscopic submucosal dissection; NET, neuroendocrine tumor; Tip-in EMR, Tip-in endoscopic mucosal resection.
R0 resection rate
All the NETs included in the EMR-P and Tip-in EMR groups were resected en bloc. The R0 resection was achieved in 31 of 32 (96.9%) NETs in the EMR-P group and 29 of 32 (90.6%) NETs in the Tip-in EMR group. The R0 resection rate difference between the two groups was −6.3% (95% confidence interval [CI]: −18.0 to 5.5), which did not demonstrate non-inferiority (Fig. 4). Detailed histological findings of the two treatment groups are presented in Table 2. The R0 resection rates for each treatment method, according to the endoscopists, are presented in Table 3. The R0 resection rate in the Tip-in EMR group for endoscopist 5 was 50.0%, but for most of the other endoscopists, the Tip-in EMR and EMR-P methods achieved > 90% R0 resection. When conducting an analysis that excludes the outcomes of endoscopist 5, whose resection rate for the Tip-in EMR technique was an outlier, and that of endoscopist 1, who was assigned a biased method, the R0 resection rate was 96.2% (25/26) for the EMR-P group and 96.4% (27/28) for the Tip-in EMR group. No statistically significant difference was observed between the two groups (p = 1.00).

R0 resection rate difference between Tip-in and precut EMR methods: modified intention-to-treat analysis. CI, confidence interval; EMR-P, precut endoscopic mucosal resection; Tipin EMR, Tip-in endoscopic mucosal resection.
Endoscopic outcome
The resection time was significantly longer in the EMR-P group than that in the Tip-in EMR group (5.0 vs. 2.9 min, p < 0.001). Intraprocedural bleeding requiring endoscopic hemostasis occurred in the Tip-in EMR group. Otherwise, no procedure-related adverse events occurred (Table 4).
DISCUSSION
In this study, we assessed the non-inferiority of Tip-in EMR, which is considered a feasible method for endoscopic resection of rectal NETs, compared to the EMR-P method. Although our study did not statistically demonstrate the non-inferiority of Tip-in EMR compared to EMR-P, both endoscopic methods showed relatively high R0 resection rates of > 90%. The R0 resection rates of both methods were similar to those of previously reported EMR-C, EMR-L, or ESD methods [14,28]. This suggests that both EMR-P and Tip-in EMR methods may be used to treat small rectal NETs. Additionally, the duration of resection time was relatively short for both methods, and no procedure-related serious adverse events occurred. Unlike EMR-C and EMR-L, the Tip-in EMR, EMR-P, and ESD methods belong to a similar spectrum of endoscopic resection procedures. Therefore, endoscopists who have expertise in Tip-in EMR, EMR-P, and ESD can choose the easier and simpler approach as an initial resection method for small rectal NETs, and if the Tip-in EMR method does not seem to be successful, the resection method can be immediately changed to EMR-P or ESD.
This study was designed to assess the non-inferiority of the Tip-in EMR method compared to that of the EMR-P method but it was inconclusive. However, numerically, the R0 resection rate for treating small rectal NETs was lower in the Tip-in EMR group than that in the EMR-P group. The most plausible explanation for this is the presence of a difference in the proficiency of the Tip-in EMR method among the endoscopists included in this study. We included endoscopists with experience in the EMR-P method for > 200 cases; however, no qualification criteria were applicable for the Tip-in EMR method because it is a more recently introduced technique for the endoscopic treatment of colorectal neoplasia [21,25–27]. Interestingly, the R0 resection rate in the Tip-in EMR group was relatively low for one endoscopist (endoscopist 5), and when the results of endoscopist 5 were excluded, the R0 resection rates were 96.6% and 96.4% in the EMR-P and Tip-in EMR groups, respectively. Although the required resection time in the Tip-in EMR method was shorter than that in the EMR-P method, various factors learned through experience may be decisive in achieving R0 resection using the Tip-in EMR method. These factors include the: 1) distance between the slit and proximal edge of the lesion; 2) direction or the degree of power when capturing the lesion by the snare; and 3) appropriate air inflation/deflation in the rectum. The technical details of Tip-in EMR to maximize R0 resection rates in rectal NETs should be investigated in future studies.
Various modified EMR methods have been applied for treating small rectal NETs, but evidence remains insufficient for determining the optimal approach. In a previous study, the EMR-C method for treating rectal NETs achieved an R0 resection rate of 94.1%, and the average procedure time was 4.2 minutes [14]. Conversely, the EMR-L method also showed a relatively high R0 resection rate of approximately 89–100%, and the average procedure time was approximately 5 minutes [29–31]. These results are comparable to those of the Tip-in EMR and EMR-P methods reported in our study. However, unlike the EMR-C and EMR-L methods, the Tip-in EMR and EMR-P procedures do not require a dedicated device. This difference is not only convenient for an endoscopist but can also reduce the procedure cost since the usage of dedicated devices for EMR-C and EMR-L is not reimbursed in Korea. Recently, underwater EMR (UEMR) has been suggested as a feasible treatment method for small rectal NETs in several retrospective studies [32–34]. The R0 resection rates of UEMR for small rectal NETs ranged between 81–100%, and two case-control studies showed no significant differences in UEMR compared to ESD [32,34]. From a practical viewpoint, the resection method for small rectal NET is selected based on the endoscopists’ preference and expertise on each procedure, available devices, and characteristics of the lesions. Additional studies should follow to elucidate how to optimize endoscopic resection methods for rectal NETs.
Our study has some limitations. First, blindly allocating treatment methods to the endoscopists was not possible. We allowed each endoscopist to decide whether or not to convert to ESD only based on their own decision. This raises the concern that factors related to the endoscopists’ skills may have influenced the outcomes. However, in the real world, the decision of the endoscopist is crucial for the selection of treatment methods, which may be acceptable. Secondly, this clinical study had a small sample size; further large scale studies are required to confirm our findings. In a subsequent clinical trial, resetting an inferiority margin based on the results of this study would be necessary. Lastly, considering the relatively small sample size, a significant imbalance was observed in the number of assigned patients among the endoscopists. This suggests that operator-related factors could have had a substantial impact on the study outcomes.
In conclusion, although we did not demonstrate the noninferiority of Tip-in EMR compared to EMR-P in the R0 resection rate of small rectal NETs, both methods achieved numerically high (> 90%) R0 resection rates. Moreover, compared to the EMR-P method, the Tip-in EMR method required a shorter procedure time, showed a similar safety profile, and could be easily switched to EMR-P whenever a concern regarding incomplete resection arose. Therefore, Tip-in EMR, as well as EMR-P, are feasible methods for treating small rectal NETs.
KEY MESSAGE
1. Both Tip-in EMR and EMR-P techniques demonstrated a high R0 resection rate in the treatment of small rectal NETs.
2. Tip-in EMR was equally safe as EMR-P and required a shorter procedure time.
3. Both of these modified EMR techniques are feasible options for treating small rectal NETs.
Acknowledgments
This study was performed by the intestinal tumor research interest group of Korean Association of the Study of Intestinal Diseases (KASID).
Notes
CRedit authorship contributions
Seung Wook Hong: methodology, investigation, data curation, formal analysis, writing - original draft; Dong-Hoon Yang: conceptualization, methodology, investigation, writing - review & editing, supervision, project administration, funding acquisition; Yoo Jin Lee: conceptualization, methodology, investigation; Dong Hoon Baek: conceptualization, methodology, investigation; Jaeyoung Chun: conceptualization, methodology, investigation; Hyun Gun Kim: conceptualization, methodology, investigation; Sung Joo Kim: investigation, data curation, formal analysis; Seung-Mo Hong: investigation, data curation, formal analysis; Dae-Seong Myung: conceptualization, methodology, investigation, supervision
Conflicts of interest
The authors disclose no conflicts.
Funding
This study was supported by a grant from the Korean Gastrointestinal Endoscopy Research Foundation (2019, Investigation Grant).