Clinical characteristics and prognostic factors of non-tuberculous mycobacterial disease in patients with rheumatoid arthritis
Article information
Abstract
Background/Aims
This study aimed to identify the clinical characteristics of patients with concurrent rheumatoid arthritis (RA) and suspected non-tuberculous mycobacterial (NTM) infections as well as determine their prognostic factors.
Methods
We retrospectively reviewed the medical records of 91 patients with RA whose computed tomography (CT) findings suggested NTM infection. Subsequently, we compared the clinical characteristics between patients with and without clinical or radiological exacerbation of NTM-pulmonary disease (PD) and investigated the risk factors for the exacerbation and associated mortality.
Results
The mean age of patients with RA and suspected NTM-PD was 65.0 ± 10.2 years. The nodular/bronchiectatic (NB) form of NTM-PD was the predominant radiographic feature (78.0%). During follow-up, 36 patients (41.9%) experienced a radiological or clinical exacerbation of NTM-PD, whereas 12 patients (13.2%) died. Combined interstitial lung disease (ILD), microbiologically confirmed NTM-PD, and NB with the fibrocavitary (FC) form on chest CT were identified as risk factors for the clinical or radiological exacerbation of NTM-PD. Hydroxychloroquine use was identified as a good prognostic factor. Conversely, history of tuberculosis, ILD, smoking, microbiologically confirmed NTM-PD, and NB with the FC form on chest CT were identified as poor prognostic factors for mortality in suspected NTM-PD.
Conclusions
ILD and NB with the FC form on chest CT were associated with NTM-PD exacerbation and mortality. Hydroxychloroquine use may lower the risk of NTM-PD exacerbation. Therefore, radiographic features and presence of ILD should be considered when predicting the prognosis of patients with RA and suspected NTM-PD.
INTRODUCTION
Rheumatoid arthritis (RA) is a systemic and chronic inflammatory joint disorder. Chronic inflammation of the synovial membrane is a critical factor in the pathophysiology of RA; it leads to the destruction of both the articular cartilage and juxta-articular bone, consequently causing disability [1]. Patients with RA have a high incidence of morbidity and mortality because of infectious diseases. This trend was identified before the advent of immunosuppressive medications that interfere with tumour necrosis factor (TNF) and other biologic disease modifying anti-rheumatic drugs (bDMARDs). In previous RA cohorts, markers of disease severity and presence of comorbidities (such as chronic lung disease, alcoholism, and diabetes mellitus) were identified as the primary risk factors for severe infections [2,3]. Additionally, long-term use of glucocorticoids is a key predictor of infection in patients with RA. These trends remain pertinent in the era of bDMARD-based treatment [3]. A recent study revealed that patients with RA are more prone to infections due to multiple factors, including high disease activity, treatment- or disease-related immunosuppression, coexisting diseases, and polypharmacy [4].
There has been a consistent increase in the occurrence and fatal impacts of non-tuberculous mycobacterial (NTM) diseases [5]. NTM diseases have a high prevalence among patients with RA, and their incidence has been increasing in South Korea [6]. The NTM group comprises members of the genus Mycobacterium, except for the Mycobacterium tuberculosis complex and Mycobacterium leprae. NTM infections can include those affecting the skin, soft tissues, bones, joints, and superficial lymph nodes; however, they are mostly associated with the lungs [7].
To date, the identified prognostic factors for the exacerbation of NTM infections in patients with RA include advanced age, systemic and respiratory comorbidities, use of glucocorticoids or biological agents (such as anti-TNF agents), and elevated levels of inflammatory markers (such as the C-reactive protein [CRP] and erythrocyte sedimentation rate [ESR]) [8–11]. However, only few laboratory-based clinical and epidemiological studies have been conducted on the risk factors for the exacerbation of NTM infections in patients with RA in Korea.
Therefore, we aimed to identify the clinical characteristics of patients with concurrent RA and NTM-pulmonary disease (PD) and determine the factors that affect their prognosis.
METHODS
Study population
This study included 91 patients with RA who were being followed up at the Rheumatology Department of the Ajou University Hospital in Korea. These patients presented with chest computed tomography (CT) findings suggestive of NTM infections between January 2002 and December 2022. The indications for a chest CT scan were abnormalities on chest radiography and persistent respiratory symptoms. Among a total of 2,248 patients with chest CT scans, NTM-PD was suspected in 91 patients. All patients fulfilled the 1987 American College of Rheumatology (ACR) criteria or the 2010 ACR/European League Against Rheumatism classification criteria for RA [12,13]. The study protocol was reviewed and approved by the Institutional Review Board of the Ajou University Hospital (AJOUIRB-DB-2023-170). The requirement to obtain consent to participate was waived due to the study’s retrospective nature.
Data collection
The medical records of patients examined between January 2002 and March 2023 were retrospectively reviewed. RA disease activity was assessed using the Disease Activity Score in 28 joints (DAS28). Furthermore, the number of tender and swollen joints, visual analogue scale pain score, and global assessment score were evaluated at RA and suspected NTM-PD diagnoses.
The age at suspected NTM-PD diagnosis was based on the date the CT scan first showed evidence of NTM infection. Clinical exacerbation of NTM-PD was considered when pulmonologists deemed patients with NTM-PD to have exacerbated signs and symptoms that necessitated admission. Radiological exacerbation was considered when the most recent imaging findings showed an aggravated condition as compared with that noted previously. Radiologically, NTM-PD was categorized as nodular/bronchiectatic (NB), fibrocavitary (FC), and unclassifiable. The NB form was characterized by the existence of multifocal bronchiectasis and clusters of small nodules. The FC form was characterized by the presence of cavitary lesions, often associated with significant involvement of the pleura overlying the affected area of the lungs [14]. The presence of interstitial lung disease (ILD) findings, such as ground-glass opacities or reticular abnormalities with honeycombing, was also determined by experienced radiologists. Differentiation between ILD exacerbation/progression and NTM-PD exacerbation was based on follow-up chest CT findings.
Statistical analysis
The baseline demographic and clinical characteristics were compared between patients with radiological or clinical NTM-PD exacerbation (i.e., the NTM-PD exacerbation group) and without NTM-PD exacerbation (i.e., the NTM-PD nonexacerbation group); a chi-squared test or Fisher’s exact test was used for categorical variables, whereas an independent t-test or Mann–Whitney U test was used for continuous variables. The laboratory results obtained at RA and NTMPD diagnoses were compared between the groups using a paired t-test. The risk factors for the exacerbation of NTM infection and associated mortality were evaluated using a Cox regression analysis; these were presented with hazard ratios (HRs) and 95% confidence intervals (CIs). All statistical analyses were performed using SPSS version 25.0 (IBM Corp., Armonk, NY, USA). p < 0.05 was considered statistically significant.
RESULTS
Baseline characteristics of patients with RA and suspected NTM-PD
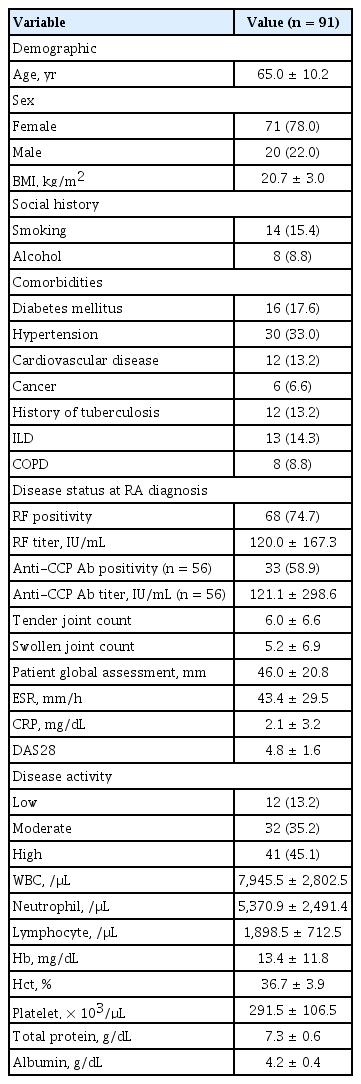
Among the 91 patients included in the study, 15 were already diagnosed with NTM-PD at RA diagnosis and 76 were newly diagnosed with NTM-PD after RA diagnosis. The mean age was 65.0 ± 10.2 years, and most were female (78.0%). The baseline patient characteristics are shown in Table 1. Hypertension (33.0%) was the most common comorbidity. Furthermore, 13 (14.3%), 8 (8.8%), and 12 (13.2%) patients had ILD, chronic obstructive pulmonary disease (COPD), and a history of tuberculosis, respectively. Furthermore, 68 (74.7%) and 33 (58.9%) patients exhibited rheumatoid factor positivity and anti-cyclic citrullinated peptide antibody positivity, respectively. At RA diagnosis, the disease activity was high (DAS28 score > 5.1), moderate (3.2 < DAS28 score ≤ 5.1), and low (2.6 ≤ DAS28 score ≤ 3.2) in 41 (45.1%), 32 (35.2%), and 12 (13.2%) patients, respectively.
The laboratory findings at RA and suspected NTM-PD diagnoses are shown in Supplementary Table 1. The ESR, DAS28 score, lymphocyte count, platelet count, and total protein levels were higher at RA diagnosis than at NTM-PD diagnosis. However, the serum CRP levels, white blood cell count, and neutrophil count did not differ significantly between the two time points.
Clinical characteristics of patients with RA and suspected NTM-PD
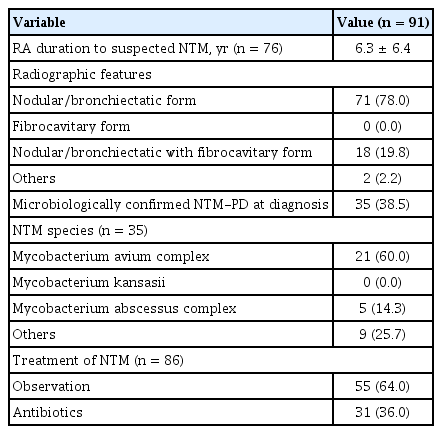
The clinical characteristics of the 91 included patients are presented in Table 2; as mentioned before, 15 and 76 patients were diagnosed with NTM-PD before and after RA diagnosis, respectively. The average duration between RA and suspected NTM diagnoses in the 76 patients was 6.3 ± 6.4 years. The NB form of NTM-PD was the predominant radiographic feature (78.0%). According to commonly used diagnostic criteria, 35 patients (38.5%) presented with two consecutive positive NTM cultures at the time of suspected NTM-PD diagnosis [15]; the Mycobacterium avium complex was the most common causative pathogen identified. Supplementary Table 2 shows the baseline characteristics of the 35 patients with RA and microbiologically confirmed NTMPD. Approximately 64% of the patients were observed without treatment; the remaining 36% were treated with NTM-targeting antibiotics.
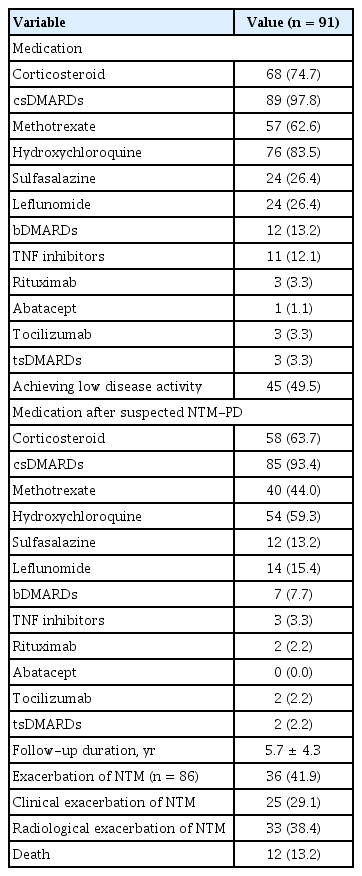
The medications and treatment outcomes of all patients are shown in Table 3. Approximately all patients (97.8%) received conventional synthetic DMARDs (csDMARDs); these were mostly hydroxychloroquine (83.5%), followed by methotrexate (62.6%). Twelve patients (13.2%) received bDMARDs; most were TNF inhibitors (12.1%). Only three patients (3.3%) received targeted synthetic DMARDs (tsDMARDs). After a suspected NTM-PD diagnosis, only three patients received TNF inhibitors, followed by rituximab (2.2%) and tocilizumab (2.2%). During follow-up, 45 patients (49.5%) achieved a low disease activity or remission state of RA (DAS28 score < 3.2), 36 patients (41.9%) experienced a clinical or radiological exacerbation of NTM-PD, and 12 patients (13.2%) died.
Exacerbation of NTM infections in patients with RA and NTM-PD
Table 4 presents a comparison of the characteristics of patients who experienced a clinical or radiological exacerbation of NTM-PD (n = 36 [41.9%]) with those of patients who did not (n = 50 [58.1%]). Five patients were lost to follow-up. Their follow-up duration from a suspected NTM-PD diagnosis was 5.7 ± 4.3 years. The number of patients with ILD was significantly higher in the NTM-PD exacerbation group than in the NTM-PD non-exacerbation group. Conversely, the number of patients with COPD and those with a history of tuberculosis did not differ significantly between the two groups. Moreover, the number of patients with a low disease activity during follow-up did not differ significantly between the two groups.
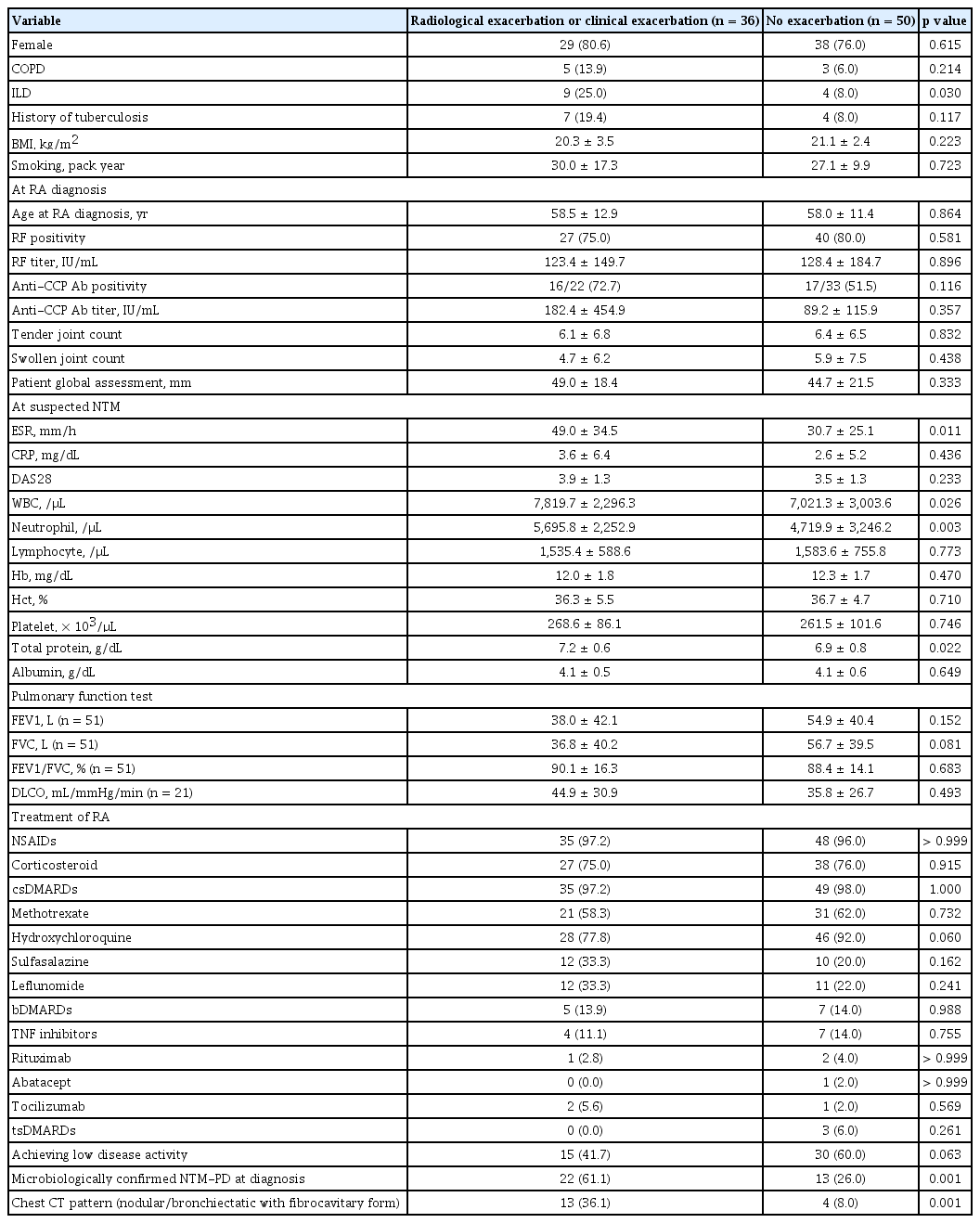
Comparison of clinical data of RA patients with exacerbation of suspected NTM-PD and those without exacerbation
At suspected NTM-PD diagnosis, the total protein levels were significantly higher in the NTM-PD exacerbation group than in the NTM-PD non-exacerbation group (7.2 ± 0.6 vs. 6.9 ± 0.8 g/dL, p = 0.022). Furthermore, the ESR was significantly higher in the NTM-PD exacerbation group than in the NTM-PD non-exacerbation group (49.0 ± 34.5 vs. 30.7 ± 25.1 mm/h, p = 0.011). Conversely, the NTM-PD exacerbation and non-exacerbation groups did not differ significantly in terms of the DAS28 score (3.9 ± 1.3 vs. 3.5 ± 1.3, p = 0.233), pulmonary function test results, and treatment patterns of RA.
We analysed the risk factors for NTM-PD exacerbation in patients with RA using Cox regression (Table 5). The risk of NTM-PD exacerbation was significantly higher in patients with ILD than in those without ILD (crude HR = 2.25, 95% CI: 1.04–4.89, p = 0.040). Furthermore, it was also significantly higher in patients with microbiologically confirmed NTM-PD at diagnosis than in those without (crude HR = 2.80, 95% CI, 1.41–5.59, p = 0.003). Hydroxychloroquine users had a significantly decreased risk of NTM-PD exacerbation as compared to non-users (crude HR = 0.38, 95% CI: 0.17–0.86, p = 0.020). However, use of other csDMARDs, biologics or tsDMARDs, and corticosteroids was not significantly associated with NTM-PD exacerbation. After adjustment, only hydroxychloroquine users were noted to have a significantly decreased risk of NTM-PD exacerbation as compared to non-users (adjusted HR = 0.33, 95% CI: 0.13–0.84, p = 0.020).
We analysed the risk factors for exacerbation of microbiologically confirmed NTM-PD in patients with RA using Cox regression (Supplementary Table 3. Patients with ILD had a marginally increased risk of NTM-PD exacerbation as compared with those without ILD (crude HR = 3.24, 95% CI: 0.98–10.77, p = 0.055).
Mortality in patients with RA and suspected NTM-PD
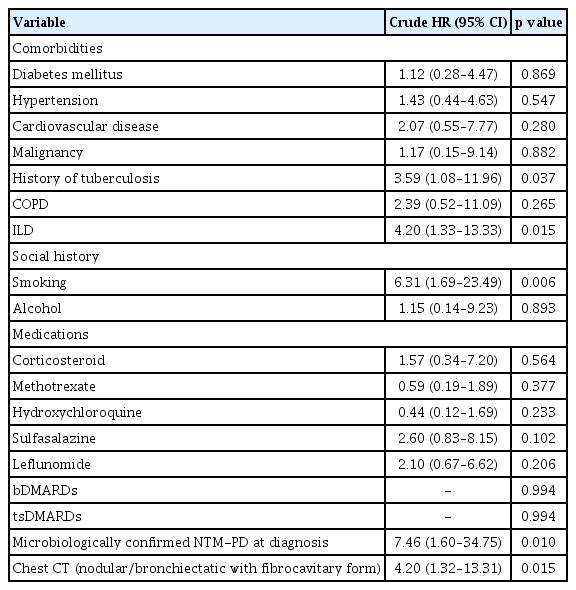
Among the 12 deaths noted, the most common cause of mortality was pneumonia (n = 9); the remaining one and two patients died of pulmonary thromboembolism and NTM-PD aggravation, respectively. The risk factors for mortality in patients with concurrent RA and suspected NTMPD are shown in Table 6. Cox regression analysis indicated that ILD (crude HR = 4.20, 95% CI: 1.33–13.33, p = 0.015), smoking (crude HR = 6.31, 95% CI: 1.69–23.49, p = 0.006), microbiologically confirmed NTM-PD (crude HR = 7.46, 95% CI: 1.60–34.73, p = 0.010), and a history of tuberculosis (crude HR = 3.59, 95% CI: 1.08–11.96, p = 0.037) were significantly related to an increased mortality risk in patients with RA with NTM-PD. In addition, NB with an FC form on chest CT was also associated with mortality (crude HR = 4.20, 95% CI: 1.32–13.31, p = 0.015). Conversely, medication usage was not significantly associated with mortality. Due to the small sample size of 12 deaths, we did not perform any adjustments.
We analysed the risk factors for mortality in patients with microbiologically confirmed NTM-PD with RA using Cox regression (Supplementary Table 4). NB with an FC form on chest CT was associated with mortality (crude HR = 12.16, 95% CI: 1.21–121.98, p = 0.034).
DISCUSSION
We performed this retrospective study using a hospital-based clinical information database to assess the risk factors of NTM-PD exacerbation and associated mortality in patients with concurrent RA and suspected NTM-PD. ILD and NB with an FC form on chest CT were identified as risk factors for the clinical or radiological exacerbation of NTM-PD. Hydroxychloroquine use was identified as a good prognostic factor; conversely, ILD, smoking, history of tuberculosis, microbiologically confirmed NTM-PD at diagnosis, and NB with an FC form on chest CT were identified as poor prognostic factors for mortality in patients with RA and suspected NTM-PD.
Yamakawa et al. [8] reported that an ESR of > 50 mm/h was a poor prognostic factor for the radiological exacerbation of NTM-PD in patients with RA. Consistent with this, the ESR was considerably higher in the NTM-PD exacerbation group than in the NTM-PD non-exacerbation group (49.0 ± 34.5 vs. 30.7 ± 25.1 mm/h, p = 0.011) [8]. In addition, compared with the NTM-PD non-exacerbation group, the NTM-PD exacerbation group had notably higher total protein levels at NTM-PD diagnosis. Individuals with inflammatory conditions (such as infections, autoimmune diseases, and cancer) experience a faster rate of decline in red blood cells; this decline leads to increased blood protein levels. Elevated plasma proteins can contribute to rouleaux formation, thereby increasing the ESR [16]. Thus, the higher protein levels and ESR observed in patients with NTM-PD exacerbation could be attributed to the more severe inflammation caused by the NTM infection at diagnosis. Furthermore, a retrospective study revealed that patients with poorly controlled RA experience worse radiological outcomes despite receiving biological therapies [17]. Conversely, in our study, the DAS28 score (representative of the RA disease activity) at suspected NTM-PD diagnosis did not differ between the NTM-PD exacerbation and non-exacerbation groups, suggesting that the severity of the inflammation caused by NTM infection (instead of the severity of RA) is associated with a worse prognosis.
ILD has been identified as a significantly more common comorbidity in individuals with RA [18]. Previous studies have revealed that patients with RA and ILD might develop tuberculosis or an NTM disease [19]. In this study, 13 patients (14.3%) had ILD as a comorbidity; ILD was identified as a significant risk factor for NTM-PD exacerbation (crude HR = 2.25, 95% CI: 1.04–4.89, p = 0.040) and mortality (crude HR = 4.20, 95% CI: 1.33–13.33, p = 0.015). In contrast, previous studies have shown that diabetes mellitus, hypertension, and COPD are significantly associated with an increased risk of NTM disease development [20]; exposure to oral corticosteroids further increases this risk [21]. In the management of RA, corticosteroids are frequently used as effective immunosuppressive drugs [20]. However, in this study, these comorbidities and corticosteroids were not associated with NTM-PD exacerbation or mortality in patients with RA and suspected NTM-PD. A retrospective study on 120 patients with NTM diseases in Helsinki [22] revealed that the mortality risk was higher in smokers than in non-smokers (HR = 1.64, p = 0.049); however, the association between smoking and mortality risk was rendered insignificant after adjusting for the underlying causes of the diseases. The present study revealed that smoking was a risk factor for mortality in patients with RA and NTM-PD (crude HR = 6.31 95% CI: 1.69–23.49, p = 0.006).
The symptoms of NTM-PD are non-specific; however, the radiological features are more indicative and generally classified into two patterns, namely bronchiectasis with nodules (referred to as the NB form) and cavitation with fibrosis (referred to as the FC form) [23]. In this study, the radiographic features included the NB form, NB with FC form, and other forms in 71 (78.0%), 18 (19.9%), and 2 (2.2%) patients, respectively. When compared with the NB form, the NB with FC form was associated with a significantly increased risk of mortality (HR = 4.20, 95% CI: 1.32–13.31, p = 0.015). This is consistent with the findings of previous studies [8,11]. The FC form, NB with FC form, and other disease forms are poor prognostic factors for mortality [8]; cavitary disease is a predictive factor for NTM-related mortality [11].
Regarding antirheumatic medications, this study showed that hydroxychloroquine usage was a good prognostic factor for radiological or clinical exacerbation. Several studies have revealed higher rates of NTM infections in patients using bDMARDs [24–26]. A meta-analysis of 70 clinical trials conducted in the US found that TNF inhibitors were associated with an increased risk of opportunistic infections, including NTM diseases (odds ratio [OR] = 3.73) [27]. However, our study did not reveal bDMARDs to be significant risk factors for NTM exacerbation or mortality in NTM-PD, consistent with previous reports that bDMARDs are not associated with a substantial risk [17,28]. A retrospective cohort study on 40 patients with NTM-PD revealed that treatment with bDMARDs was not associated with a significantly worse mortality [28]. Other studies have further shown no association between bDMARDs and exacerbation of NTM infections [17,28], consistent with our findings. Hydroxychloroquine is a relatively safe and well-tolerated drug for RA treatment. It was the most frequently prescribed csDMARD among our patients; according to the national patient sample data from the Health Insurance Review and Assessment Service (2016), it was the second most (30.1%) frequently prescribed csDMARD in Korea after methotrexate (45.4%) [29]. A case-control study on 56,269 patients with RA found that hydroxychloroquine could increase the risk of NTM infection in older patients with RA (OR = 1.62) [25]. However, in our study, we observed a lower risk of exacerbation of NTM infections in patients administered hydroxychloroquine. Hydroxychloroquine reportedly exhibits favourable outcomes in various infectious conditions. For example, studies have demonstrated both its ability to attenuate the release of pro-inflammatory cytokines in sepsis models and its effectiveness in treating brucellosis when included in different treatment regimens [30,31]. Furthermore, in patients with systemic lupus erythematosus who experienced serious infections, hydroxychloroquine was found to be protective against infection-related mortality [32]. These findings suggest a potential preventive role of hydroxychloroquine in certain infections. Nonetheless, it is important to acknowledge that data regarding the association between hydroxychloroquine and NTM-PD exacerbation are limited, underscoring the need for further investigations with larger sample sizes.
This study had several limitations. First, it was a retrospective study conducted at a single referral centre, which may limit the generalizability of its findings. Second, the study had a relatively small sample size, which may affect the statistical power and precision of its results. Third, the possibility of selection bias cannot be excluded. For instance, being a tertiary hospital, the prevalence of comorbidities and the severity of RA or NTM-PD may be higher in our patients than in the general population. Moreover, drawing a causal inference on the association between treatment and outcome based on observational data is challenging, even with quasi-randomization methods (such as propensity score matching). It is important to acknowledge the potential influence of immortal time bias when comparing csDMARD-users and bDMARD-users in RA; this bias can lead to a survival advantage for bDMARD users. Fourth, this study included patients with suspected NTM-PD for analysis. Several studies have revealed cases wherein bronchial brushing or washing and repeated culture failed to confirm NTM microbiologically; in some cases, physicians do not proceed with biopsy upon considering the associated risks [33,34]. Therefore, in this study, we included patients with suspected NTM based on CT findings.
In conclusion, physicians should be cautious about NTMPD exacerbation in patients with RA with high ESR and protein levels. NTM-PD exacerbation and mortality were associated with concomitant ILD and NB with FC on chest CT. Furthermore, treatment with hydroxychloroquine reduced the risk of NTM-PD exacerbation.
KEY MESSAGE
1. Combined ILD and the NB form with FC form on chest CT were associated with exacerbation and mortality in patients with RA with suspected NTMPD.
2. Hydroxychloroquine could be a preventive factor for NTM infections in patients with RA and suspected NTM-PD.
Acknowledgments
We would like to express our gratitude to Dr. Sun-Kyung Lee for assisting us with data management and statistical analysis. Additionally, we acknowledge the assistance of ChatGPT in English language editing during the manuscript revision process.
Notes
CRedit authorship contributions
Hyemin Kim: conceptualization, methodology, resources, investigation, data curation, formal analysis, validation, software, writing - original draft; Soyoung Lee: conceptualization, methodology, resources, investigation, data curation, formal analysis, validation, software, writing - original draft; Ji-Won Kim: methodology, resources, investigation, data curation, supervision; Ju-Yang Jung: methodology, resources, investigation, data curation, validation, software, supervision; Chang- Hee Suh: methodology, resources, investigation, data curation, supervision; Hyoun-Ah Kim: conceptualization, methodology, resources, investigation, data curation, formal analysis, validation, software, writing - review & editing, visualization, supervision, project administration
Conflicts of interest
The authors disclose no conflicts.
Funding
None