Sarcopenia in chronic kidney disease: from bench to bedside
Article information
Abstract
Sarcopenia is a condition characterized by a loss of muscle mass and function. In chronic kidney disease (CKD), where a chronic catabolic state exists, sarcopenia commonly occurs through various mechanisms, resulting in muscle wasting and decreased muscle endurance. Sarcopenic patients with CKD have high morbidity and mortality rates. Indeed, the prevention and treatment of sarcopenia are mandatory. An imbalance between protein synthesis and degradation in muscle and increased oxidative stress and inflammation persist in CKD and induce muscle wasting. In addition, uremic toxins negatively affect muscle maintenance. A variety of potential therapeutic drugs targeting these muscle-wasting mechanisms in CKD have been investigated, but most of the trials focused on aged patients without CKD, and none of these drugs have been approved for the treatment of sarcopenia so far. Further studies on the molecular mechanisms of sarcopenia in CKD and targets for potential therapeutics are needed to improve the outcomes of sarcopenic patients with CKD.
INTRODUCTION
Sarcopenia is a condition characterized by a loss of muscle mass and function (either muscle strength or physical performance) and generally develops with age [1]. When first used, the term sarcopenia was used to describe an age-related loss of muscle mass only [2]. The definition of sarcopenia has been updated and now includes muscle function. The most widely used definition of sarcopenia nowadays is proposed by the European Working Group on Sarcopenia in Older People (EWGSOP) 2. The EWGSOP2 considers low muscle mass as a key characteristic of sarcopenia, low muscle quantity and quality to confirm the diagnosis and poor physical performance to indicate the severe sarcopenia [3]. The operational definition of sarcopenia proposed by EWGSOP2 is presented in Table 1. Sarcopenia is associated with poor health-related quality of life, including organ dysfunction [4,5] and is a significant risk factor for some cancers [6].
However, in addition to aging, several underlying conditions, including malnutrition, low physical activity, specific drugs and diseases can also cause sarcopenia. In patients with chronic kidney disease (CKD), where a chronic catabolic state exists, muscle wasting and decreased muscle endurance occur, and sarcopenia commonly occurs [2]. Compared to the aging-related sarcopenia where protein degradation is not changed, CKD-related sarcopenia is related to the increased muscle protein degradation and protein energy wasting (PEW), cachexia are usually present in patients with CKD [7,8]. The prevalence of sarcopenia using different definitions (mostly EWGSOP1 [9] and EWGSOP2 [3] criteria) ranged from 5.9% [10] to 14% [11] in CKD patients without kidney replacement therapy, 13.7% [12] to 42.2% [13] in patients on hemodialysis (HD), and 4% [14] to 15.5% [15] in patients on peritoneal dialysis. Indeed, sarcopenic patients with CKD have significantly worse physical performance [11,16], a higher risk of disability [13], an increased risk of intradialytic hypotension during HD [17], increased mortality [10,18-21], and cardiovascular events [22,23]. Therefore, the diagnosis and interventions to treat sarcopenia in patients with CKD are important to improve outcomes.
Risk factors for sarcopenia in patients with CKD include both modifiable factors (malnutrition [11,12,18,19,22,24-26] and low body mass index [16,19,27,28]) and non-modifiable factors (age [19,24,26,27,29], male sex [27,30], diabetes mellitus [12,19,27,29], longer dialysis duration [28] and dialysis modality [29]). Because of the differences between the aging-related and CKD-related sarcopenia as described above, the treatment goals are also different. In patients with aging-related sarcopenia, restoring mobility and quality of life is the main goals. In patients with CKD-related sarcopenia where muscle wasting and PEW are more prominent, recovering nutritional status to improve the response to the treatment of the CKD is more important [7]. Aerobic and resistance exercises show inconsistent positive effects on sarcopenia but still play a major role as interventions for the prevention and treatment of sarcopenia in patients with CKD [31-34]. Additionally, nutritional interventions [35-39], optimized dialysis, and correction of acidosis [40,41] are important strategies [42]. However, there is no consensus on the unified methods of both exercise and nutritional interventions, which limits the practical approach to sarcopenia in patients with CKD. Therefore, understanding the pathophysiology and molecular mechanisms of sarcopenia in CKD and developing potential therapeutic agents are important. In this review, we aimed to address pharmacologic and nutritional interventions in which the targets were derived from the molecular mechanisms of sarcopenia in CKD.
BODY TEXT
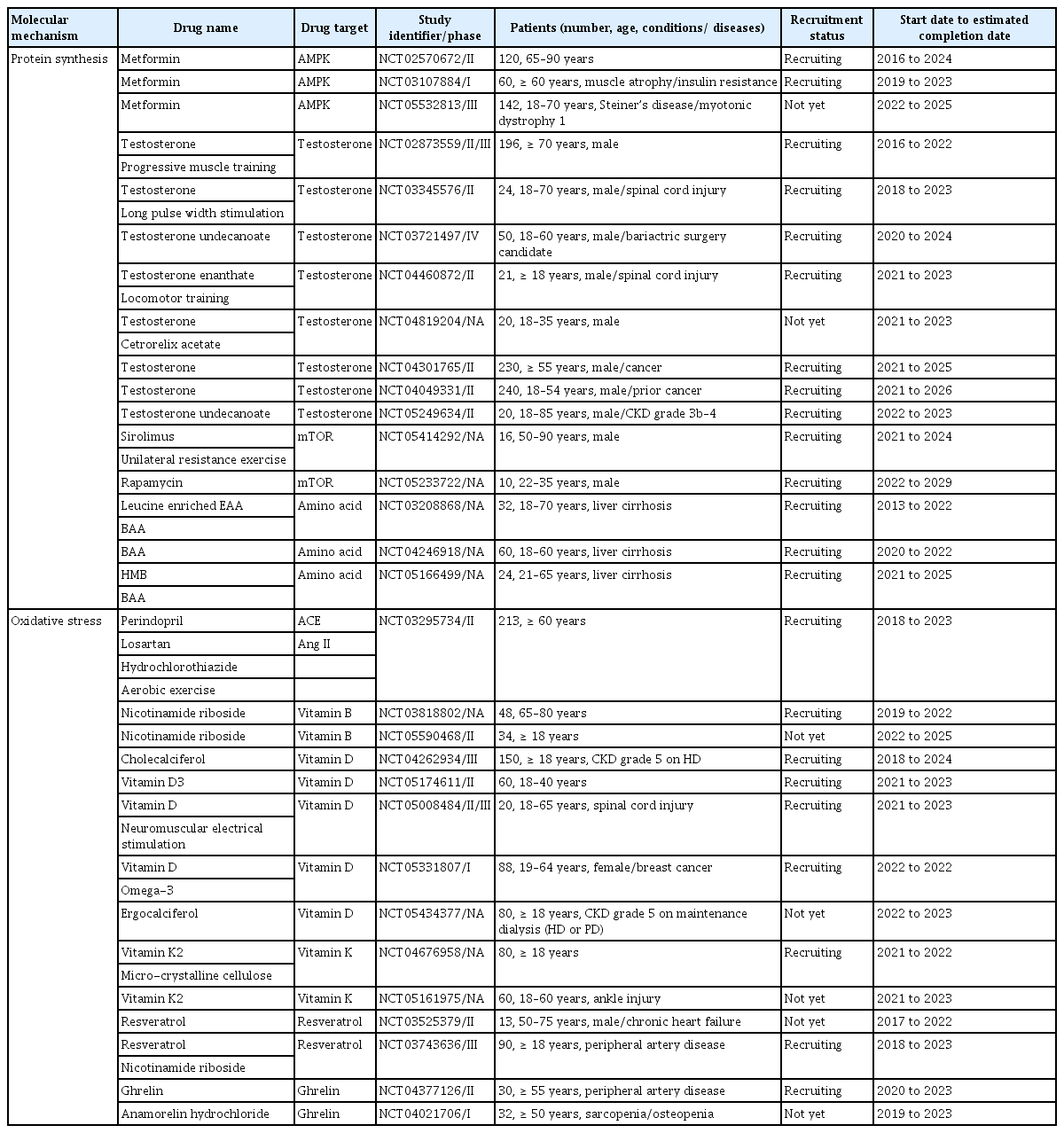
Chronic catabolic conditions persist in CKD and induce an imbalance between protein synthesis and degradation, resulting in muscle wasting [43,44]. Chronic inflammation and uremic toxin-induced muscle wasting are other important factors associated with sarcopenia in CKD. This review involves elucidating the mechanisms of sarcopenia associated with CKD in terms of protein synthesis and degradation of muscle, oxidative stress with inflammation, and the effect of uremic toxins, and describing the available drugs or nutritional supplements for each mechanism (Fig. 1). The ongoing clinical trials designed to investigate the drug’s effect on muscle were researched on October 2022 through ClinicalTrials.gov and are presented in Table 2.

Molecular mechanisms of muscle wasting in chronic kidney disease and targeted drugs or supplements. CKD, chronic kidney disease; ROS, reactive oxygen species; RAAS, renin-angiotensin-aldosterone system; IL, interleukin; TNF, tumor necrosis factor; IGF, insulin- like growth factor; SARM, selective androgen receptor modulator; IS, indoxyl sulfate; Rc, receptor; ActRIIB, activin receptor type IIB; OAT, organic anion transport; PI3K, phosphatidylinositol 3-kinase; FoxO, forkhead box protein O; mTOR, mammalian target of ramamycin; mTORi, mammalian target of ramamycin inhibitor; MuRF1, muscle RING-finger protein-1; AHR, aryl hydrocarbon receptor; NADPH, nicotinamide adenine dinucleotide phosphate.
PROTEIN SYNTHESIS IN MUSCLE
Alterations in protein synthesis have been consistently observed in animal models and some patients with CKD [45-50]. Through the mammalian target of ramamycin (mTOR) and inactivation of the Forkhead box protein O (FoxO), the insulin or insulin-like growth factor (IGF)-1-phosphatidylinositol 3-kinase-Akt pathway increases protein synthesis in muscle [51-55]. Impaired insulin tolerance induces muscle atrophy [56] and IGF-1 treatment suppresses FoxO expression [57]. In addition, testosterone binds to the androgen receptor (AR) and regulates myogenic gene expression by stimulating Akt/mTOR complex 1 (mTORC1) and suppressing FoxO-targeted gene expression. As a result, it promotes protein synthesis and inhibits protein degradation [58,59]. Drugs relative to the mechanisms of protein synthesis include metformin, testosterone, selective androgen receptor modulators (SARM), and mTOR inhibitors. Also, the supplementation of the amino acid is another treatment option to stimulate the muscle protein synthesis.
Metformin
Diabetic nephropathy is the leading cause of CKD in Korea and the United States [60-62]. Diabetes mellitus is a major risk factor for sarcopenia in patients with both CKD and non-CKD [12,19,27,29,63] and interestingly, sarcopenia is an independent risk factor for diabetic nephropathy in type 2 diabetes [64]. Hyperglycemia contributes to the loss of both muscle mass and function by increasing insulin resistance, inflammatory cytokines, and accumulation of glycation end-products [65-67]. Several classes of anti-diabetic agents have shown a positive effect on improving energy metabolism in muscles in vivo [68-72] and this review is focused on the most extensively investigated drug, metformin. Metformin is a commonly prescribed drug for type 2 diabetes, and by activating AMP-activated protein kinase (AMPK), it induces the expression of muscle hexokinase and glucose transporters, mimicking the effects of extensive exercise training [73]. In addition, metformin is known to increase the follicular fluid IGF-1 levels in patients with polycystic ovary syndrome [74] and this finding suggests the promotion of protein synthesis in muscles by metformin. However, metformin treatment did not increase muscle mass or longevity in either the sedentary or exercise mouse groups [75]. Metformin also blunted increases in mTORC1 signaling in response to progressive resistance exercise training and negatively impacted the hypertrophic responses to exercise in healthy older adults [76]. In line with these findings, a meta-analysis showed that metformin did not affect exercise capacity [77]. Although the previous disappointing results of the studies, several clinical trials are recruiting the patients to investigate the effect of metformin on muscle mass and function (Table 2).
Testosterone and SARM
Testosterone plays an important role in the maintenance of muscle mass and function via the aforementioned molecular mechanisms. More than 60% of men with CKD have testosterone deficiency and uremic hypogonadism [78-80]. In mice, testosterone improved skeletal muscle regeneration and prevented muscle atrophy [81,82]. Several trials involving testosterone have consistently shown a positive effect of the drug in increasing muscle mass and function in older male patients [83-86]. However, various side effects of testosterone, including cardiovascular events, prostate hyperplasia, and lower urinary tract symptoms, occurred during the trials [83,87]. Clinical trials using testosterone undecanoate, which showed no risk of prostate cancer or cardiovascular disease [88] are recruiting patients and one trial (NCT05249634) is recruiting the patients with CKD (Table 2). SARMs function as anoints/antagonists of AR, and by their selective action, SARM therapy has fewer off-target side effects [89]. Several SARMs, such as MK-0773, GTx-024, and GSK2881078, increased lean body mass in healthy older individuals, but MK-0773 and GTx-024 failed to improve physical performance [90-93].
mTOR inhibitor
mTORC1 promotes protein synthesis by phosphorylating two key effectors, p70S6 kinase 1 and eIF4E binding protein [94]. Acute activation of mTORC1 signaling in vivo promotes muscle hypertrophy [95], but in the chronic state, both inhibition and hyperactivation of mTORC1 result in muscle atrophy. Long-term inhibition of mTOR by rapamycin induced insulin resistance of muscle in rats [96]. In a retrospective study in which the patients received mTOR inhibitors for at least 6 months, the drugs significantly decreased the skeletal muscle area and lean body mass [97]. Moreover, mTOR hyperactivation has been observed in both aged rodent and human muscles [98]. In a mouse model of muscle dystrophy, hyperactive mTORC1 signaling was observed, and the mTORC1 inhibitor rapamycin improved skeletal muscle function [99]. Although evidence of hyperactivation of mTORC1 in muscles is scarce in CKD, acute and chronic kidney injury constitutively activates mTOR signaling in kidney fibroblasts, leading to kidney damage [100]. Therefore, mTOR inhibitors are potential drugs for the treatment of sarcopenia in CKD. Based on these findings, two clinical trials investigating the impact of mTOR inhibition on muscle are currently recruiting patients (Table 2).
Amino acids
Amino acids are classified to the essential amino acid (EAA) and non-EAA. Among the EAAs, the branched-chain amino acids (BCAA) [101,102] and leucine [103-105] are known to induce stimulation of muscle protein synthesis. In CKD, various combined conditions (inflammation, catabolic illnesses [106], acidosis [43,107,108], nutritional loss to dialysate [109-111], endocrine disorders such as resistance to insulin [112], growth hormone [113], and IGF-1 [114], hyperparathyroidism [115]) can lead to PEW where body stores of protein and energy fuels are decreased [116]. Therefore, the plasma and cellular levels of the BCAA and leucine are commonly low in CKD [115]. Since protein restriction is essential to minimize uremic toxicity and delay progression of the kidney disease, BCAA and leucine supplements are effective to improve the sarcopenia while reducing the total amount of the protein intake in CKD patients [117]. Both in rat and elderly patients, administration of BCAA and leucine are effective to improve muscle protein synthesis [118-121]. In HD patients with malnutrition, the EAA supplements improved appetite, increased plasma albumin levels and enhanced muscle strength [36,37,122]. However, β-hydroxy-β-methylbutyrate, a metabolite of leucine showed no benefit on body composition in HD patients [123]. Currently, several clinical trials are recruiting patients with chronic liver disease to investigate the effect of amino acid supplementation on sarcopenia (Table 2).
PROTEIN DEGRADATION IN MUSCLE
In CKD, protein degradation increases via increased expression of atrophy-inducing genes (atrogen) and atrophy-related biomarkers [51,124-127]. Myostatin (growth differentiation factor 8) is an autocrine inhibitor of muscle growth and is mainly produced in skeletal muscles [128,129]. It binds to activin type 2 receptors on the muscle fiber membrane and subsequently recruits and activates activin type 1 receptor B and transforming growth factor β to phosphorylate Smad 2/3 [130]. In addition, it reduces Akt signaling, regulates the Akt/mTOR pathways, and suppresses the FoxO pathway [131]. Through this pathway, myostatin promotes protein degradation in the muscle and functions as a negative regulator of muscle mass [132]. The myostatin maturation process and extracellular binding proteins, such as follistatin, also regulate the myostatin pathway, and follistatin functions as a myostatin antagonist [133]. Myostatin increases in patients with CKD, and several factors, including low physical activity, oxidative stress and inflammation, uremic toxins, angiotensin II (Ang II), metabolic acidosis, and glucocorticoids, maybe the contributors [51,134-136]. Drugs that target the myostatin pathway include myostatin inhibitors, activin receptor antagonists, and follistatin-based drugs. Most trials investigating the effects of drugs on sarcopenia have recruited patients without CKD.
Myostatin inhibitor
The upregulation of muscle myostatin was observed both in a CKD rodent model and in patients with CKD [135,137]. In CKD mice, an anti-myostatin peptibody suppressed circulating inflammatory cytokines and reversed the loss of body weight and muscle mass [135]. Landogrozumab (LY-2495655), a humanized monoclonal antibody for myostatin, increased lean mass and showed a tendency to improve functional measures of muscle power in older patients [138]. Another monoclonal anti-myostatin antibody, trevogrumab (REGN-1033), showed a tendency toward increased muscle size in only a few patients with muscular dystrophy and demonstrated good safety and tolerability [139].
Activin receptor antagonist
In a mouse model, bimagrumab, a specific monoclonal antibody that binds to the activin type 2A/2B receptor, significantly promoted skeletal muscle hypertrophy [140,141]. A phase II clinical trial with bimagrumab showed the effect of the drug on increasing thigh muscle mass and grip strength and improving mobility in patients with sarcopenia [142]. A subsequent phase II/III trial was completed in 2018, and data analysis is ongoing [143]. In patients with type 2 diabetes and obesity, bimagrumab resulted in the loss of fat mass, gain of lean mass, and metabolic improvements [144]. Ramatercept, a soluble form of the activin type 2 receptor, significantly increased the cross-sectional area of type I and II muscle fibers in a mouse model [145], but in its phase II trial, serious non-muscle-related adverse events of the drug were observed, and the trial was terminated [146].
Follistatin fusion protein and gene therapy
Intramuscular injection of FST288-Fc, a follistatin fusion protein, induced the growth of targeted muscles [147] and systemic administration of monovalent follistatin-like 3-Fc-fusion protein induced muscle fiber hypertrophy and increased muscle mass in a mouse model [148]. Another follistatin-based fusion protein, ACE-083, also induced localized skeletal muscle hypertrophy and increased focal force generation in a mouse model [149]. However, in a phase II trial of ACE-083, treatment increased muscle mass but did not improve functional outcomes [150]. Associated virus (AAV) serotype 1. Follistatin, which acts as a natural myostatin antagonist, significantly increases muscle mass and strength in a mouse model of muscular dystrophy [151]. FS344, an isoform of follistatin by AAV 1, improved ambulation in patients with Becker muscular dystrophy or sporadic inclusion body myositis [152-154].
OXIDATIVE STRESS AND INFLAMMATION
Oxidative stress and inflammation are features of CKD [155] and they also induce muscle wasting. Through the nuclear factor kappa-light-chain-enhancer of activated B cells pathway, reactive oxygen species (ROS)-induced tumor necrosis factor (TNF)-α activates myostatin expression [137] and increased inflammatory cytokines, such as TNF-α and interleukin (IL)-6, causing muscle atrophy in patients with CKD [156-158]. Owing to their antioxidant effects, angiotensin-converting enzyme (ACE) inhibitor, Ang II type I receptor blocker (ARB), vitamins, resveratrol, and its anti-inflammatory effect, ghrelin are potential therapeutic drugs and supplements for sarcopenia in CKD.
Renin-angiotensin-aldosterone system (RAAS)
The RAAS plays a role in systemic physiology and is responsible for blood pressure control, maintenance of fluid homeostasis, and electrolyte balance [159]. Along with the major contributions of RAAS to these mechanisms, aberrant signaling through RAAS in CKD also influences muscle wasting [160]. The protease renin cleaves angiotensinogen and forms angiotensin I (Ang I), and ACE cleaves Ang I to produce Ang II. The three most investigated membrane receptors for RAAS hormone peptides are the Ang II type 1 receptor (AT1R), Ang II type 2 receptor, and the mitochondrial assembly receptor (MASR). These receptors are expressed in various tissues, including smooth muscle and skeletal muscle fibers [161,162]. When Ang II binds to AT1R on the cell membrane, the classical RAAS signaling pathways are activated. By transducing the signals to downstream secondary messengers, AT1R signaling produces ROS and contributes to muscle wasting [160]. Angiotensin-(1-7), the principal hormone in the non-classical RAAS pathway, activates MASR and inhibits AT1R activation [163]. Therefore, disrupting the classical RAAS pathway through inhibition of ACE or blocking of AT1R and promoting MASR are potential therapeutic targets to reduce muscle wasting.
ACE inhibitors and ARB are the most commonly prescribed antihypertensive drugs in patients with CKD because of their effect on slowing the decline in kidney function, decreasing urine protein excretion, and adverse cardiovascular outcomes [164-167]. Along with renoprotective effects, these drugs are expected to inhibit muscle atrophy by blocking Ang II production. Treatment of hypertension with ACE inhibitors showed a slower decline in muscle strength and mobility [168] and higher muscle mass of the lower limb than with other antihypertensive drugs [169]. It also increased the IGF-1 levels in older patients [170]. However, in a recently published randomized controlled trial to determine the efficacy of leucine and/or perindopril in improving physical performance or muscle mass in older patients with sarcopenia, neither leucine nor perindopril showed this effect [171]. In the case of ARB, losartan improved muscle remodeling, protected against disuse atrophy [172], improved mobility, and reduced inflammation and oxidative stress in sarcopenic mice [173]. However, losartan showed no effect in preventing mobility loss in older adults [174]. In addition, there was no significant effect in preventing muscle strength loss in pre-frail older patients with losartan treatment (NCT01989793, completed in 2016). One clinical trial (NCT03295734) is recruiting older patients to investigate the effect of perindopril on muscle function compared to losartan or hydrochlorothiazide while all patients will participate in a structured aerobic exercise intervention (Table 2).
MASR agonists attenuate muscle atrophy by activating MASR and inhibiting the downstream signaling of the AT1 receptor. AVE 0991, an MASR agonist, showed multiple attenuated muscles wasting in mice with cancer cachexia [175]. A phase II trial investigating the safety and tolerability of the MASR agonist BIO101 measured gait speed, several body mass indicators, and power in older patients (NCT03452488). The study was completed but no significant results were reported.
Vitamins and resveratrol
Vitamin B is a cofactor with methyl donors regulating the level of homocysteine. Uremia-induced hyperhomocysteinemia occurs in patients with CKD and is associated with poorer outcomes [176-178]. Several studies demonstrated that high homocysteine levels are also linked to impaired muscle strength [179,180] and physical performance [181,182] in older patients. A 2-year randomized controlled trial of vitamin B12 and folic acid supplementation showed a positive effect on gait speed, but not on muscle strength [183]. Two clinical trials investigating the vitamin B3 are ongoing (Table 2). Vitamin C is a potential water-soluble antioxidant, and its effects have been demonstrated in many in vitro experiments [184-188]. Plasma vitamin C concentration declines with kidney function [189] and additional loss of this component into the dialysate occurs in patients on HD [190]. Higher vitamin C intake was associated with higher skeletal muscle mass and power in free-living women [185]. Therefore, clinical trials investigating the effect of vitamin C supplementation on muscle in patients with CKD seem promising though not conducted yet.
Because vitamin D has a structure homologous to cholesterol, it may be regarded as an antioxidant, and this effect has been suggested to have a non-calcemic role [158]. Vitamin D deficiency in CKD is common and is associated with low bone formation rate, bone mineral density, and muscle atrophy [191]. In a mouse model, vitamin D3 reduced the extent of lipid peroxidation and induced superoxide dismutase activity; these effects were similar to those of vitamin E [158]. In older patients, vitamin D supplementation increased muscle mass and strength [192,193]. The effect of vitamin D was enhanced in older patients with vitamin D deficiency [194]. Several clinical trials are actively recruiting patients to investigate the effect of vitamin D on muscle and two trials are recruiting the patients on maintenance dialysis (Table 2). Vitamin K acts as a cofactor of γ-carboxylation of some proteins [195,196] and one of the γ-carboxylated proteins, AR has an important role in protein synthesis of the muscle as described above [197]. CKD patients have subclinical vitamin K deficiency [198,199]. Several clinical studies demonstrated that high vitamin K was associated with better physical performance, suggesting the beneficial effect of vitamin K in muscle quality [200-203]. To investigate the benefit of vitamin K supplementation on muscle, two clinical studies are designed and one study is currently recruiting the patients (Table 2).
Resveratrol is a natural phenolic compound found in many foods, such as grapes, blueberries, and peanuts. Resveratrol has been shown to have antioxidant and anti-inflammatory properties [204,205]. Resveratrol improved skeletal muscle mass and function and prevented sarcopenia in a rat model [206-208]. In addition, resveratrol improved exercise performance and physical endurance in a mouse model [209,210]. However, in a trial of humans, resveratrol impaired exercise training-induced improvements by reducing oxidative stress and inflammation markers in skeletal muscles [211]. Two clinical trials evaluating the clinical efficacy of resveratrol in improving skeletal muscle in patients with chronic heart failure or peripheral artery disease are being conducted (Table 2).
Ghrelin
Ghrelin is an acylated peptide that stimulates growth hormones and subsequently stimulates feeding [212]. In addition to its effects on appetite regulation, ghrelin has been shown to exert anti-inflammatory effects. There are major forms of circulating ghrelin, acylated and des-acyl ghrelin. Acylated ghrelin increases food intake and des-acyl ghrelin induces negative energy balance [213,214] The levels of des-acyl were elevated in CKD patients [215]. Ghrelin and a synthetic ghrelin receptor agonist significantly decreased the expression of IL-1 receptor-I transcript in the brain and thus improved lean body mass retention in a rat model of cancer cachexia [216]. In CKD rats, treatment with ghrelin and two ghrelin receptor agonists (BIM-28125 and BIM-28131) resulted in decreased muscle protein degradation and circulatory inflammatory cytokines, thus increasing food intake and improving lean body mass [217]. Ghrelin and the ghrelin agonist anamorelin have been shown to increase food intake and muscle mass in cancer patients [218-221]. One clinical trial investigating the effect of ghrelin is recruiting patients with peripheral artery disease (NCT04377126). Capromorelin, a ghrelin receptor agonist, increased lean mass and physical performance in sarcopenic elderly patients [222]. A phase II trial designed to determine the effect of MK-0677, a growth hormone secretagogue, on lean body mass in CKD stage 4/5 patients was withdrawn because the investigators could not obtain drug supply from the manufacturer (NCT01343641).
UREMIC TOXINS
Uremic toxins increase in serum along with a decline in kidney function, and the negative effect of accumulated uremic toxins on muscle wasting is a specific mechanism of CKD [223]. Protein-bound uremic toxins, including p-Cresyl sulfate and indoxyl sulfate (IS), have been investigated for their effects on muscle wasting. These toxins are taken up by cells through an organic anion transporter [224,225]. In mice, p-Cresyl sulfate alters the insulin signaling pathway by suppressing insulin-induced phosphorylation of Akt, resulting in insulin resistance [226]. IS induces metabolic alterations via nuclear factor (erythroid-2-related factor)-2 in skeletal muscle [227]. IS also enhances the production of muscle atrophy-related genes like myostatin and atrogin-1 and increases ROS and inflammatory cytokines [228,229]. In addition, it acts as an aryl hydrocarbon receptor (AHR) ligand, and AHR works as a component of the ubiquitin ligase complex [230,231]. Through these mechanisms, IS induces skeletal muscle wasting and is a potential therapeutic drug target [229,232]. AST-120, an absorbent capsule used to remove circulating IS, significantly reversed the negative changes in the skeletal muscle by reducing circulating IS in CKD mice [232]. In a phase IV trial, AST-120 showed modest benefits in gait speed change and quality of life, but the changes were not significant in patients with CKD [233].
CONCLUSIONS
This review elucidated the brief molecular mechanisms of sarcopenia associated with CKD and the potential therapeutic drugs and nutritional supplements for sarcopenia categorized by each mechanism. Various mechanisms, including an imbalance in protein synthesis and degradation, increased oxidative stress and inflammation, and uremic toxins, contribute to muscle wasting and result in sarcopenia in CKD. Some potential therapeutic drugs have been investigated, and promising drugs are under ongoing clinical trials. Further clinical trials testing the effects of drugs in patients with CKD and more studies unveiling the potential molecular treatment targets for sarcopenia are needed to improve the outcomes of sarcopenic patients with CKD.
Notes
CRedit authorship contributions
Da Woon Kim: writing - original draft, writing - review & editing; Sang Heon Song: conceptualization, project administration, writing - review & editing
Conflicts of interest
The authors disclose no conflicts.
Funding
None