The role of roxadustat in chronic kidney disease patients complicated with anemia
Article information
Abstract
The incidence of chronic kidney disease (CKD) is increasing worldwide and the current prevalence rate is 13.4%. There are > 120 million CKD patients in China and this number is expected to increase. One of the main abnormalities in patients with CKD and kidney impairment is decreased synthesis of erythropoietin (EPO), which causes anemia and affects iron metabolism. The probability of developing is higher in anemia patients with CKD than in the general population, and the incidence increases as kidney function decreases. Deficient EPO production by the kidney is the most important cause of renal anemia. Notably, anemia in patients with CKD has multiple causes, such as bleeding caused by platelet dysfunction, iron deficiency due to digestive and absorption disorders of the gastrointestinal tract, and shorter red blood cell life. Anemia is also a leading cause of hospitalization in patients with CKD. A new oral medication to treat renal anemia, the hypoxia-inducible factor prolyl hydroxylase inhibitor called roxadustat (FG-4592), regulates iron metabolism and promotes erythropoiesis. This drug has a therapeutic effect on patients with CKD. Roxadustat showed advantages over EPO in clinical experiments. This review summarizes the mechanisms of action, clinical applications, effectiveness, and safety of roxadustat.
INTRODUCTION
Chronic kidney disease (CKD) has diverse causes and affects the health of people worldwide. Approximately 10% of the world’s population suffers from CKD, and this rate is expected to increase in the future [1–3]. Anemia is a serious complication of CKD, and a variety of factors can cause renal anemia (Fig. 1). More than 90% of patients on dialysis have anemia [4], which leads to an increase in morbidity and mortality [5]. Recent studies have shown that transfusion rates decrease, and clinical outcomes improve, in CKD patients when anemia is effectively treated [6]. Renal anemia requires aggressive treatment, as it increases the probability of blood transfusion or hospitalization, and can be fatal [5,7]. Traditional treatments, including iron agents and erythropoiesis-stimulating agents (ESAs), are the main treatment options for renal anemia. Iron replacement is an effective alternative to increase iron stores and hemoglobin levels. However, the safety of long-term intravenous administration of iron agents is unknown [8]. Intravenous medications, such as intravenous iron, are poorly tolerated and have several side effects. Long-term use of ESAs is related to an increased risk of cardiovascular diseases and infections, such that ESAs are a second-line drug treatment for renal anemia [9,10].
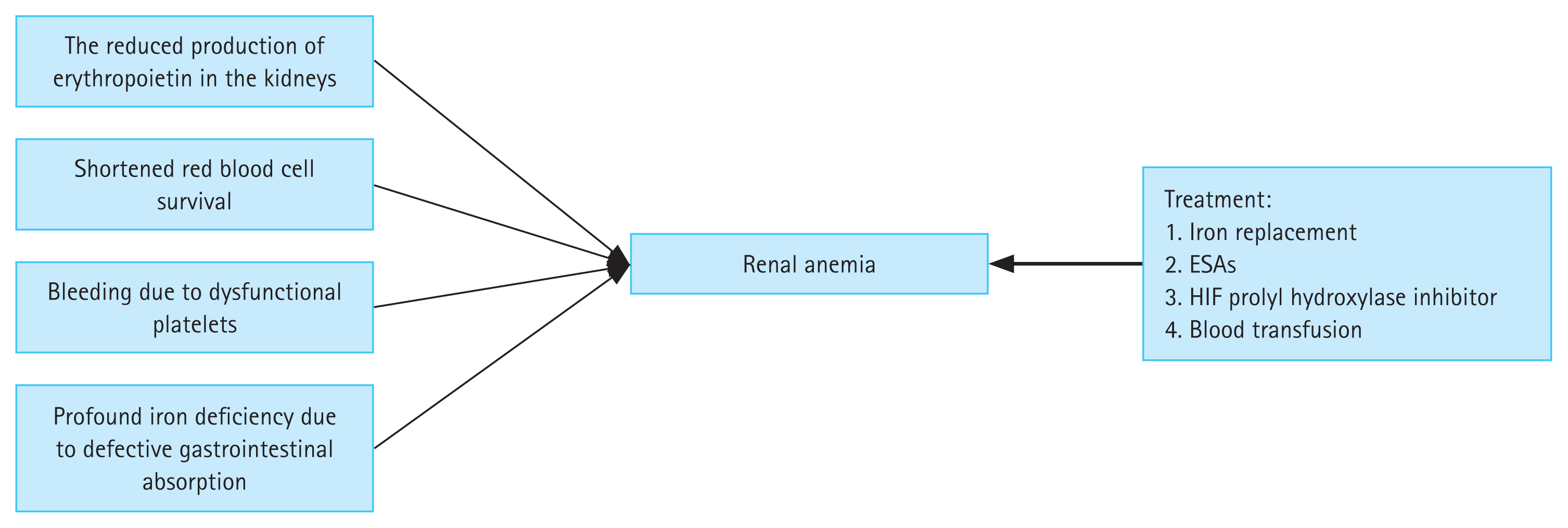
Etiology and treatment options for renal anemia. ESA, erythropoiesis-stimulating agents; HIF, hypoxia-inducible factor.
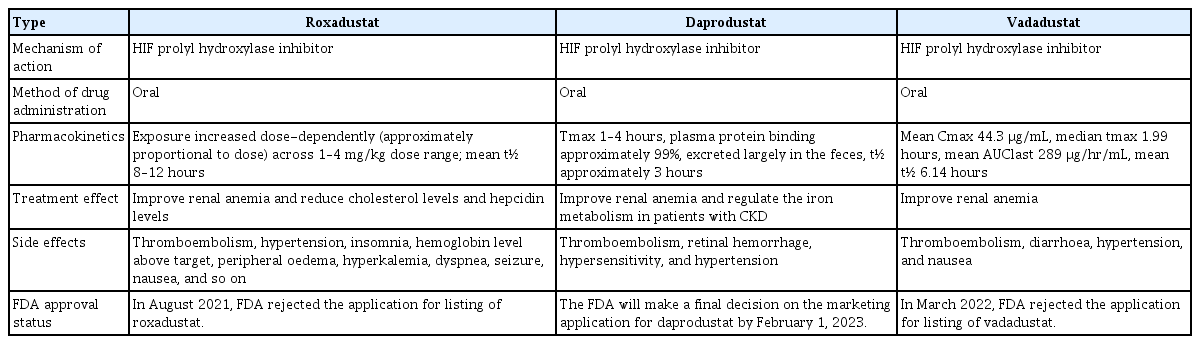
Several hypoxia-inducible factor (HIF) prolyl hydroxylase inhibitors have been used clinically to improve renal anemia, among which roxadustat, daprodustat, and vadadustat have been best-studied. All three drugs significantly improve renal anemia, but differ in half-life, side effects, and Food and Drug Administration (FDA) approval status; we briefly summarize these differences in Table 1.
Roxadustat is a prolyl hydroxylase inhibitor that increases HIF transcriptional activity by stabilizing HIF-α subunits. The increased transcriptional activity promotes erythropoiesis by activating related enzymes and receptors, including erythropoietin (EPO) and iron-related enzymes and receptors. Many options are available for treating renal anemia, among which promoting hematopoiesis by increasing HIF activity has emerged as a promising new treatment modality. HIF prolyl hydroxylase inhibitors treat anemia in CKD patients by increasing the EPO level and modulating inflammation and iron handling. In particular, these inhibitors decrease the hepcidin level [11]. Prolyl hydroxylase enzymes sense oxygen tension, according to which the HIF level changes [12]. Prolyl hydroxylase enzyme activity decreases with oxygen tension, which causes HIF-α subunit packing and increases HIF transcriptional activity; this leads to an increase in EPO expression, as well as iron recycling and absorption [13]. Some phase 2 trials of other HIF prolyl hydroxylase inhibitors have reported hyperkalemia as a side effect [14,15]. Larger phase 3 trials have provided a more complete picture, indicating that hyperkalemia may be a class effect of HIF prolyl hydroxylase inhibitors. These observations will help guide the design of future trials and clinical monitoring. We have compared the occurrence of hyperkalemia among the different HIF prolyl hydroxylase inhibitors in Table 2 [16–20].
MECHANISMS OF ACTION OF ROXADUSTAT AND DIFFERENCES BETWEEN ROXADUSTAT AND ESAs
Roxadustat mimics the body’s natural response to hypoxia, thus improving anemia in a reversible manner. Intermittent administration of roxadustat is used to treat anemia [21] in CKD patients and exerts a lasting effect [22–24]. Roxadustat is administered three times per week; the half-life of the drug is 10 hours, which is sufficient to restore HIF transcriptional activity to baseline levels. Hypoxia activates target genes, thereby promoting erythropoiesis during this period [25,26].
Patients starting dialysis in rural areas have much lower hemoglobin levels than those in urban areas, and heart failure and mortality rates are higher [27]. Before 1989, i.e., before the approval of ESAs, anemia in patients with CKD could only be relieved by blood transfusion, and EPO improved their condition. Anemia decreases quality of life, causes left ventricular hypertrophy and increases mortality. ESAs effectively increase the hemoglobin level to the ideal range (9 to 11 g/dL), thus reducing the adverse effects of anemia [28,29]. However, several clinical studies have reported that the efficacy of ESAs is unsatisfactory and they do not improve clinical outcomes; in fact, they can increase the risk of death and stroke [30–32]. Why does normalizing the hemoglobin level not reverse the effects of anemia in patients with CKD? A post hoc analysis showed that higher doses of ESAs were correlated with worse clinical outcomes; however, no clear pathophysiological mechanisms have been established [33,34]; more intensive research is needed to identify the specific mechanisms.
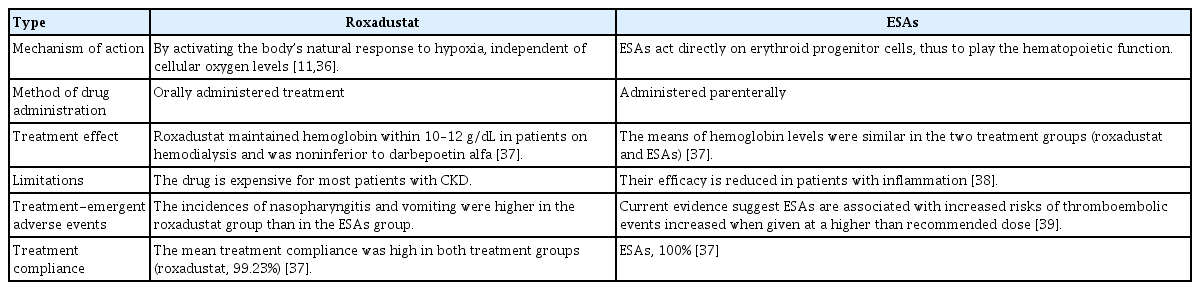
In dialysis patients, roxadustat was not inferior to ESAs [20]; the mean change in hemoglobin levels from baseline to weeks 23 and 27 was in fact greater with roxadustat administration than with ESAs, although the difference was not significant. Unlike ESAs, roxadustat affects iron metabolism, which not only increases transferrin levels but also maintains serum iron levels, thereby preventing any reduction of transferrin saturation. Roxadustat reduces total cholesterol and low-density lipoprotein (LDL) cholesterol more than ESAs. In addition, roxadustat reduces the hepcidin level. Hyperkalemia and upper respiratory tract infections were detected as side effects in the roxadustat group, while hypertension was more common in the ESA group. The same study showed that roxadustat was more likely to cause hyperkalemia than ESAs, which may explain why high doses of ESAs contributed to poor clinical outcomes. Although reports of hyperkalemia may be affected by the bias inherent in open-label trials [35], a double-blind study of nondialysis patients also showed that patients receiving roxadustat were more prone to hyperkalemia and metabolic acidosis compared with those in the ESA group [19]. Global phase 3 trials have been undertaken to provide further data on roxadustat.
The cardiovascular safety of roxadustat was demonstrated in preliminary trials reported by the American Society of Nephrology Kidney Week in 2019, and the incidence rates of hyperkalemia in roxadustat, ESA and placebo groups were similar in these large global trials. Because several clinical experiments have reported an increased probability of developing hyperkalemia when using this agent, caution is needed in patients prone to hyperkalemia. The differences between roxadustat and ESAs are summarized in Table 3 [11,36–39].
ROXADUSTAT IN PATIENTS WITH CKD UNDERGOING HEMODIALYSIS
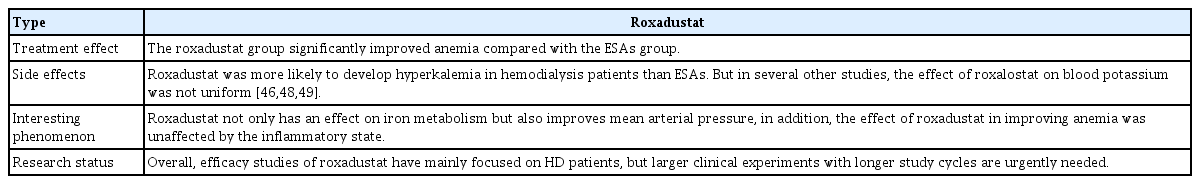
Previous studies on roxadustat have focused on hemodialysis (HD) patients. One study of dialysis patients reported that roxadustat was no less effective than ESAs [20]. Roxadustat significantly improved anemia compared with ESAs. In addition, roxadustat affects iron metabolism by increasing transferrin levels and enhancing total iron-binding capacity, as well as stabilizing serum iron levels. These effects may be related to the ability of roxadustat to alter the biomarker levels of iron. Interestingly, roxadustat enhances mean arterial pressure compared with ESAs. HD patients taking roxadustat were more likely to develop hyperkalemia than those taking ESAs. However, that study was limited in terms of sample size and duration; larger and longer international phase 3 studies are therefore required. Nevertheless, this phase 3 trial showed noninferiority of roxadustat compared with ESAs for treating anemia in patients undergoing HD.
Several studies have found that higher ESA doses do not improve the low hemoglobin levels of patients with high C-reactive protein (CRP) levels relative to those with normal levels. This result is consistent with previously published findings of inflammation suppression by ESAs [40,41]. In contrast, in accordance with phase 2 studies of roxadustat, the effect of roxadustat on hemoglobin was not dependent on inflammatory status. Another study that assessed inflammation status according to CRP [23] reported that hemoglobin levels improved independent of the baseline CRP level when patients were in an inflammatory state. The effect of roxadustat on hemoglobin was not related to the baseline CRP level, which explains why roxadustat is effective in CKD patients even if they are in an inflammatory state [42–44]. As such, roxadustat may be an appropriate option for patients in a high inflammatory state with low reactivity to ESAs undergoing HD. Furthermore, it was speculated that the stability of serum iron in the roxadustat group was associated with reduced hepcidin levels, which allows iron absorption by the gut and enhances iron conversion to transferrin in macrophages [45]. However, the influence of ethnicity may preclude extrapolation of some of the experimental results to other populations.
Some new hypotheses have been proposed in the past 2 years in association with deeper research on roxadustat and confirmation of its ability to improve anemia and regulate iron metabolism. Roxadustat reduces LDL cholesterol in CKD dialysis patients compared to ESAs, which is beneficial for the cardiovascular and cerebrovascular systems [46,47]. However, the specific mechanism of the reduction in LDL cholesterol is still unclear and may be related to the expression of an insulin-induced gene [47]. Contrary to the view that roxadustat is more likely to cause hyperkalemia in HD patients than ESAs, Provenzano et al. [48] found a lower probability of hyperkalemia in a roxadustat group compared with an ESA group. However, the serum potassium levels in the roxadustat and ESA groups were comparable during the treatment period in two other studies [46,49]. The details of roxadustat treatment in CKD patients undergoing HD are summarized in Table 4.
ROXADUSTAT IN CKD PATIENTS UNDERGOING PERITONEAL DIALYSIS
A few studies have investigated the effects of roxadustat on peritoneal dialysis (PD) patients, and most of the clinical studies have been reported in China. For example, one experiment included a patient undergoing PD, so that study did not provide statistical support for the efficacy of roxadustat in patients treated with continuous ambulatory PD [50]. In another study of 12 patients undergoing PD, the efficacy of roxadustat was independent of inflammatory status and baseline iron repletion status, and the drug also reduced serum hepcidin levels [44].
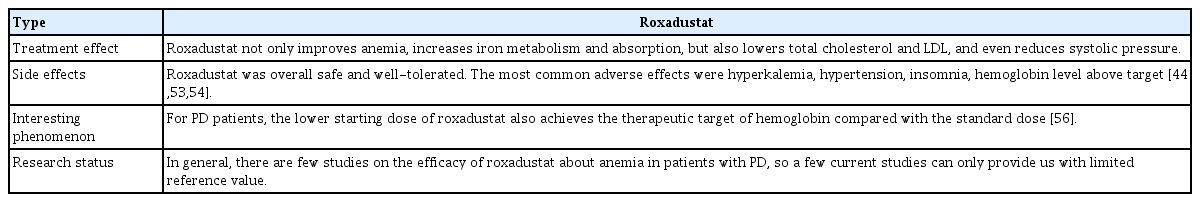
In a clinical study of the treatment of anemia in CKD patients undergoing PD, which was the earliest report of roxadustat use in this population [51], oral roxadustat not only increased hemoglobin, but also maintained it within the target range regardless of whether it was directly orally administered without ESA therapy or the patient had been switched from ESA to roxadustat. Furthermore, a decrease in serum hepcidin levels was detected in all of the treatment groups in that study, consistent with the conclusions of previous studies on roxadustat [22,24,44,52]. This finding also suggests increased iron metabolism and absorption [51]. These randomized, phase 3, multicenter, open-label studies have improved our understanding of the efficacy and safety of roxadustat in PD patients, and were followed by similar studies carried out worldwide, particularly in China. In addition to the effects mentioned above, several recent studies of PD patients have shown that roxadustat decreases total cholesterol and LDL [53], and even reduces systolic pressure [54]. Roxadustat has less effect on residual renal function than ESAs. Roxadustat was safe and well-tolerated overall by patients undergoing PD. The most common adverse effects were hyperkalemia, hypertension, insomnia, a hemoglobin level above the target value [50–52], baseline electrocardiogram abnormalities, transient arteriovenous block, and transient liver function abnormalities [44]. Also, some patients experience itching, although it is believed that this is not caused by roxadustat. Two patients reportedly developed severe hypertension, but this was resolved via dose reduction of roxadustat [55].
Few studies have been conducted on the efficacy of roxadustat in anemic patients undergoing PD, and the reference value of current studies is limited. Therefore, larger multicenter, long-term clinical studies are needed to provide more definitive conclusions. The details of roxadustat treatment in CKD patients undergoing PD are summarized in Table 5 [44,53,54,56].
ROXADUSTAT IN NONDIALYSIS PATIENTS WITH CKD
Roxadustat increases hemoglobin levels in nondialysis patients with CKD regardless of intravenous iron administration [23,51]. Roxadustat significantly increases hemoglobin levels in patients diagnosed with CKD and is more effective than placebo in patients who are not on dialysis, and noninferior to ESAs in patients undergoing HD. Roxadustat is an effective treatment option for these patients; however, due to limitations in sample size and study length, the efficacy of roxadustat requires further exploration [19,20]. Similar to dialysis patients, roxadustat significantly improved anemia status and reduced hepcidin and total cholesterol levels. Hepcidin increases iron transport protein synthesis and iron absorption by the gut, which is downregulated by hypoxia and the stabilization of HIF. Hepcidin plays a key regulatory role in iron mobilization and absorption from macrophages and hepatocytes [57,58]. The mechanism underlying the cholesterol-lowering effect of roxadustat is unclear, but it may be related to acetyl coenzyme A, which is required for the first step in cholesterol synthesis and degradation of 3-hydroxy-3-methyl-glutaryl coenzyme A reductase [59,60].
Statins reduce the risk of cardiovascular disease in CKD patients who are not on dialysis [61]; if roxadustat can lower cholesterol, it may benefit cardiovascular vessels. However, similar to dialysis patients, hyperkalemia and metabolic acidosis remain common adverse reactions of roxadustat in patients not undergoing dialysis. A clinical study performed in China confirmed the efficacy of roxadustat for treating anemia and maintaining hemoglobin [19], but the effects of the drug on iron metabolism may have been underestimated [62]. A systematic review and meta-analysis of dialysis and nondialysis patients reported that roxadustat significantly increased hemoglobin levels, and reduced those of hepcidin and ferritin, compared with placebo and ESAs. Transferrin levels also improved, but this was more pronounced in nondialysis patients [63].
In the past 2 years, clinical studies of roxadustat in nondialysis patients have shown that, in addition to improving renal anemia and reducing LDL cholesterol and hepcidin, roxadustat also decreases the need for blood transfusion and intravenous iron supplementation compared with ESAs and placebo [64–67]. Similar to dialysis patients, the efficacy of roxadustat for improving anemia was not affected by the inflammatory status of nondialysis patients, in contrast to ESAs [65,66]. Overall, the safety of roxadustat was similar to that of ESAs and placebo. More common adverse events include end-stage kidney disease, pneumonia, hypertension, and urinary tract infection [65]. The details of roxadustat treatment in nondialysis CKD patients are summarized in Table 6.
DRUG METABOLISM AND SAFETY OF ROXADUSTAT
Roxadustat is excreted in the feces and urine. Some scholars believe that CKD affects the nonrenal clearance of roxadustat [50] via the accumulation of uremic toxins, which leads to changes in the expression or activity of drug-metabolizing enzymes and transporters [68,69]. The effective t1/2 of roxadustat is a suitable parameter to predict drug accumulation better than the t1/2 because it has multicompartmental kinetics [45]. Furthermore, HD/hemodiafiltration does not affect the clearance of roxadustat, suggesting that roxadustat can be administered before or after dialysis [50].
Despite the remarkable efficacy of roxadustat in the treatment of renal anemia, the safety concerns cannot be ignored. However, previous clinical studies revealed no difference in treatment-emergent adverse effects (TEAEs) between roxadustat and control or placebo groups. The most frequent adverse effects of patients in the roxadustat group were thromboembolism, hypertension, insomnia, hemoglobin levels exceeding the target, peripheral edema, hyperphosphatemia, dyspnea, seizure, and nausea. Furthermore, the probability of treatment discontinuation because of TEAEs was higher in the roxadustat group [46].
Potassium levels vary among clinical studies. Chen et al. [19,20] reported that the most common adverse effects in their roxadustat group were hyperkalemia and metabolic acidosis. We speculate that this is related to the intracellular hypoxia-like environment caused by the HIF-stabilizing effect of roxadustat. Roxadustat as a HIF prolyl hydroxylase inhibitor modulates the switch from aerobic to anaerobic metabolism. The consequence of increased glycolysis is tissue acidification associated with the overproduction of lactic acid. Acidosis causes potassium to move from the inside to outside of the cell, which leads to hyperkalemia [62]. However, several recent clinical studies have drawn different conclusions regarding the effect of roxadustat on serum potassium. No difference in serum potassium distribution was detected between the two groups in some studies [46,49,66], while hyperkalemia was higher in the ESA than roxadustat group in some clinical trials [48,65]. The effect of roxadustat on serum potassium is not mentioned in the drug instructions. Based on the results of clinical studies, hyperkalemia is considered an off-target rather than class effect of roxadustat.
In August 2021, an FDA advisory committee reported that while roxadustat was comparable to ESAs in terms of efficacy, it posed a risk of severe thromboembolic events, among other adverse effects. Recent clinical studies also showed that roxadustat posed a higher risk of thromboembolic events than placebo and ESAs, regardless of dialysis status, such as arteriovenous fistula thrombosis, deep vein thrombosis, pulmonary embolism, and arteriovenous access thrombosis [45,46,48,64,67]. A meta-analysis published in 2022 also noted that roxadustat poses a higher risk of deep venous thrombosis in nondialysis-dependent CKD patients [70]. However, these studies did not explain the mechanism underlying the thromboembolism risk. According to an exploratory analysis by the FDA advisory committee, greater increases and decreases in hemoglobin were associated with higher rates of thromboembolic events. The developers of roxadustat speculate that thromboembolic risk may be lowered by using a lower starting dose, although this remains to be confirmed. As mentioned above, a lower starting dose of roxadustat in PD patient achieves the target hemoglobin level more effectively than the standard dose [56]. Therefore, in our next study we will explore the relationship between a lower starting dose of roxadustat and thromboembolic risk. Furthermore, roxadustat increases vascular endothelial growth factor (VEGF) levels; this raises concerns about its effects on tumors because VEGF has effects on angiogenesis, vascular permeability, and tumor formation [71]. However, transcription of the VEGF gene is regulated by HIF-1α and HIF-2α binding to hypoxia response elements [72].
CONCLUSIONS
Roxadustat is a new drug to treat renal anemia that increases hemoglobin and serum transferrin levels, as well as intestinal iron absorption, and also reduces the level of hepcidin. Moreover, it is effective in both nondialysis-dependent and dialysis-dependent CKD patients. Roxadustat improves patient compliance, but the long-term safety and efficacy require further study; thus, larger prospective multicenter clinical studies are needed.
Acknowledgments
This study was supported by funding from the National Natural Science Foundation of China (82000703); Science and technology development fund of Affiliated Hospital of Xuzhou Medical University (XYFY2020038). We are sincerely grateful to every member of the research team and to our hospital staff for their support and contribution.
Notes
No potential conflict of interest relevant to this article was reported.