The efficacy and safety of DW1601 in patients with acute bronchitis: a multi-center, randomized, double-blind, phase III clinical trial
Article information
Abstract
Background/Aims
DW1601, an oral fixed dose combination syrup composed of DW16011 and Pelargonium sidoides, was developed to enhance the symptom relief effect in patients with acute bronchitis. We evaluated the efficacy and safety of DW1601 compared to DW16011 or P. sidoides for treatment of acute bronchitis using a randomized, double-blind, placebo-controlled, multi-centre trial design.
Methods
A total of 204 patients with acute bronchitis was randomized 1:1:1 to receive DW1601 (n = 67), DW16011 (n = 70), or P. sidoides (n = 64) for 7 days. The primary outcome was efficacy of DW1601 compared to DW16011 or P. sidoides in reducing the total bronchitis severity score (BSS) at day 4 of treatment. Secondary endpoints were changes in total and symptom-specific BSS, response rate and patient satisfaction rate. Safety analysis was assessed at day 7.
Results
At 4 days after medication, decrease of total BSS from baseline was significantly greater in the DW1601 group than in the DW16011 group (−3.51 ± 0.18 vs. −2.65 ± 0.18, p = 0.001) or P. sidoides group (−3.56 ± 0.18 vs. −2.64 ± 0.19, p < 0.001). In addition, the BSS total score at day 7 and the BSS cough and sputum component scores at days 4 and 7 were significantly more improved with DW1601 treatment compared with the DW16011 group or P. sidoides group. Participants treated with DW1601 showed higher rates of response and satisfaction than control groups (response rate, DW1601, 100% vs. DW16011, 85.7% vs. P. sidoides, 85.9%; satisfaction rate, DW1601, 92.6% vs. DW16011, 82.9% vs. P. sidoides, 81.2%). Significant adverse events were not observed in the DW1601 group.
Conclusions
DW1601 is superior to DW16011 or P. sidoides in improving symptoms of acute bronchitis.
INTRODUCTION
Acute bronchitis is an inflammation of the bronchi by infectious organisms (e.g., viruses, some bacteria). Cough and sputum production are common symptoms in patients with acute bronchitis [1–4]. Cough and sputum production is defence mechanisms in the respiratory tract, but severe symptoms can decrease quality of life [5–7]. During the coronavirus disease 2019 (COVID-19) pandemic, people with cough and sputum are mistaken for patients with COVID-19 and experience decreased quality of life due to the social stigma [8,9]. Therefore, control of cough and sputum are important to improve quality of life, especially during the COVID-19 pandemic.
The treatment for acute bronchitis is symptomatic treatment for cough and sputum production because acute bronchitis can resolve spontaneously without antibiotics or antiviral agents. The main components of treatment are antitussives, expectorants, and bronchodilators [5,6,10–13]. Single-pill combination therapy incorporating different classes of drugs into one pill provides several advantages such as higher efficacy rates, reduced pill burden, and improved patient adherence. Therefore, single-pill combinations have usually been used in patients with acute bronchitis. DW1601, an oral fixed dose combination syrup composed of DW16011 and Pelargonium sidoides, was developed to enhance the symptom relief in patients with acute bronchitis. DW16011 is oral fixed dose combination syrup consisting of the expectorant ammonium chloride, the antihistamine chlorpheniramine maleate, the antitussive dihydrocodeine tartrate, and the bronchodilator di-methylephedrine HCl. This drug was commercially available and was used in patients with acute bronchitis in Republic of Korea. In addition, P. sidoides, an herbal remedy that can relieve symptoms of acute bronchitis in adults and children, was included [14–19]. In this study, we hypothesized that DW1601 is more effective for relieving cough and sputum production in patients with acute bronchitis compared with a single syrup using DW16011 or P. sidoides.
The purpose of this study was to investigate the efficacy of DW1601 compared to DW16011 or P. sidoides for symptom relief in patients with acute bronchitis.
METHODS
Study overview
This study was a multi-centre, randomized, double-blind, parallel, active controlled phase III clinical trial to evaluate the efficacy and safety of DW1601 compared to DW16011 or P. sidoides in patients with acute bronchitis. This study was conducted in six institutions in Republic of Korea between December 2018 and December 2019. This study consisted of a screening visit (visit 1), a baseline visit (visit 2), a Day 4 ± 1 day visit (visit 3), and an end of treatment visit on Day 7 ± 1 day (visit 4). The primary outcome was efficacy of DW1601 compared to that of DW16011 or P. sidoides in reducing the bronchitis severity score (BSS) at 4 days after medication in patients with acute bronchitis [20,21]. The secondary outcome was to assess the efficacy of DW1601 compared to DW16011 or P. sidoides in reducing total BSS at 7 days and in relief of each symptom through BSS assessment at 4 and 7 days post-treatment and to assess patient satisfaction with improvement of symptoms at 4 and 7 days post-treatment. This study protocol was uploaded to Clinicaltrial.gov before the beginning of the study (Number: NCT04260555). The study protocol, available in Supplementary Methods, was approved by the Institutional Review Boards of all applicable institutions (Korea University Guro Hospital, IRB number: 2018GR0369; Kangbuk Samsung Hospital, KBSMC2018-11-010). This study was conducted in accordance with the Declaration of Helsinki and the international conference on harmonization-good clinical practice. We ensured protection of patient privacy and anonymity. All patients provided written informed consent before enrollment, and the study was monitored by an independent data and safety monitoring committee.
Patients
The inclusion criteria were age ≥ 19 years, experienced acute bronchitis and persistent cough and purulent sputum production within 48 hours before randomization, and patients with total score of BSS ≥ 5 points and sputum BSS ≥ 1 point at the stage of randomization. The composite BSS was determined by the sum of severity ratings, 0 (absent), 1 (mild), 2 (moderate), 3 (severe), or 4 (very severe) for the five bronchitis-related features of cough, sputum, dyspnea, chest pain, and crackle [20,21].
The exclusion criteria were severe lung disease (e.g., bronchiectasis, lung cancer, interstitial lung disease, pneumonia, pulmonary tuberculosis, chronic obstructive pulmonary disease, asthma, chronic bronchitis, and emphysema); antibiotic use because of present infection; sleep apnea; elevated liver enzyme (≥ 3 times the normal range); severe renal dysfunction (glomerular filtration rate < 30 mL/min); uncontrolled diabetes mellitus (random plasma glucose ≥ 250 mg/dL); uncontrolled hypertension (systolic blood pressure ≥ 160 or diastolic blood pressure ≥ 100 mmHg); active peptic ulcer and gastrointestinal bleeding; coagulopathy; cataract(s); advanced malignancy; urinary obstruction due to benign prostate hypertrophy; significant heart disease (e.g., Class III/IV heart failure, pulmonary hypertension, peripheral artery disease, corrected QT interval > 450 seconds on electrocardiograph); antibiotics, antiviral agents, steroids, anticoagulants, or other medications for relieving cough and sputum during the study period; monoamine oxidase (MAO) inhibitors (e.g., antidepressant, antipsychotics, or anti-Parkinson’s drugs) within 2 weeks before randomization; smokers (≥ 15 cigarettes/day); glucose intolerance; pregnant or lactating women; drug allergy for substances associated with study drugs; loss to follow-up; use of drugs that can cause drug-drug interactions with study drugs; and failure to provide informed consent.
Randomization
The Data and Safety Monitoring Board performed randomization based on an interactive web response system. Patients received placebo or drug using a double-blind, 1:1:1 ratio of DW16011, P. sidoides, and DW1601 through a random table. A stratified block randomization method was used.
Study procedures
Of the screened patients, those eligible were randomly assigned into one of three groups: DW16011, P. sidoides, or DW1601. Patients in each group received the assigned medication three times per day for 7 days: (1) DW1601 arm (DW1601 20 mL + DW16011 placebo 20 mL + P. sidoides placebo 9 mL); (2) DW16011 arm (DW1601 placebo 20 mL + DW16011 20 mL + P. sidoides placebo 9 mL); (3) P. sidoides arm (DW1601 placebo 20 mL + DW16011 placebo 20 mL + P. sidoides 9 mL). The efficacy and safety of each drug were evaluated at 4 and 7 days post-treatment by the Data and Safety Monitoring Board. After the end of treatment, patients were interviewed by telephone to monitor the safety of the drug for 5 days (Supplementary Fig. 1). We recommended that enrolled patients take medication at above 75% of the total drug dosage and assessed medication compliance through the return of unused medications. Patients who took the medication below 75% of the total drug dosage were excluded from the final analysis. For safety monitoring, the Data and Safety Monitoring Board investigated adverse events, adverse drug reactions, and serious adverse events in enrolled patients from the time of enrolment to the follow-up period (for 5 days after end of treatment).
Outcomes
The primary outcome was efficacy of DW1601 compared to that of DW16011 or P. sidoides in reducing total BSS in patients with acute bronchitis after 4 days of medication. The secondary outcomes were change in BSS on day 7 compared to baseline, change in symptom-specific BSS on day 4 and day 7, response rate (proportion of subjects whose BSS dropped below 3 points or whose BSS decreased by more than 7 points compared to baseline), and response rate reported by investigators. We also investigated self-reported patient satisfaction as very satisfied, satisfied, neutral, dissatisfied, or very dissatisfied; satisfaction rate was determined by the percentage of subjects whose treatment was rated either “very satisfied” or “satisfied.”
Statistical analysis
The number of subjects was calculated with reference to the results of past clinical trials having the same indications and the same endpoints. The minimum sample size assuming two-sided α of 0.05, 90% power (total power over 80%), and drop-out rate of 20% was 204 (68 per group). For efficacy and safety evaluation, subjects were randomized into three groups in a 1:1:1 ratio by stratified block randomization using site as the stratification variable. In efficacy evaluation analysis, full analysis set was used as the main analysis, and per-protocol set was used as the sub-analysis. The descriptive statistics of the endpoints of each group are shown, and unless otherwise noted, every test was conducted with a two-sided test with a significance level of 5%. Primary endpoint analysis required comparisons between the test group and each control group by analysis of covariance (ANCOVA) with total baseline BSS as a covariate. The 95% two-sided confidence interval (CI) and p value of difference of least squares mean are shown, and the test group was superior to each control group if the upper limit of the CI was less than zero. Secondary endpoints were difference of total BSS on day 7 and difference of BSS of each symptom on day 4 and day 7 and were analysed by ANCOVA with total BSS at baseline as a covariate. Response rates at day 4 and day 7 and satisfaction rate, and frequency and percentage of each administration group are presented, and the difference among the three groups was analysed by Pearson’s chi-square test or Fisher’s exact test.
RESULTS
Enrolment of patients
During the study period, 204 patients with acute bronchitis were randomized to the DW1601, DW16011, or P. sidoides group. We performed this study with 68 patients randomized to DW1601, 70 patients randomized to DW16011, and 66 patients randomized to P. sidoides. Of the 68 patients randomized to DW1601 and the 66 patients randomized to P. sidoides, two were excluded because of duplicate registration. In addition, of the 66 patients randomized to P. sidoides, one was excluded because of missing BSS measurements after randomization. Therefore, the data of the 67 patients randomized to DW1601, 70 patients randomized to DW16011, and 64 patients randomized to P. sidoides were included in the final analysis (Fig. 1).
Baseline characteristics of patients
The baseline characteristics of the patients are shown in Table 1. There were no differences in baseline characteristics among groups. The mean age was 40 years, and 34.3% (69/201) were male. The mean body mass index was 23 kg/m2, and there were 162 (80.6%) non-smokers. The symptom duration of cough and sputum production was almost 32 hours, and 43 patients (21.4%) had comorbid diseases. Patient compliance was almost 98%, and there was no difference in compliance among the three groups.
Primary outcome
The primary outcome was efficacy of DW1601 compared to DW16011 or P. sidoides alone in reduction of total BSS in patients with acute bronchitis at 4 days after medication. The decrease in total BSS from baseline was significantly greater in the DW1601 group (indicating lesser severity of acute bronchitis) than in the DW16011 group (−3.51 ± 0.18 vs. −2.65 ± 0.18, p = 0.001) or the P. sidoides group (−3.56 ± 0.18 vs. −2.64 ± 0.19, p < 0.001) (Fig. 2, Supplementary Table 1). The average difference in BSS change from predose to post-dose between DW1601 and DW16011 was −0.86 ± 0.26, (95% CI, −1.37 to −0.35), while that between DW1601 and P. sidoides was −0.92 ± 0.26 (95% CI, −1.44 to −0.40). The upper limits of the 95% CI prove the superiority of DW1601 over DW16011 or P. sidoides (p = 0.001, p < 0.001, respectively).
Secondary outcomes
At 7 days after medication, decrease of total BSS from baseline was significantly greater in the DW1601 group than in the DW16011 group (−5.38 ± 0.14 vs. −4.46 ± 0.14, p < 0.001) or P. sidoides group (−5.44 ± 0.16 vs. −4.66 ± 0.17, p = 0.001) (Fig. 2, Supplementary Table 1). In addition, the change in BSS of cough and sputum production at day 4 and day 7 was significantly more reduced in the DW1601 group compared with the DW16011 group or P. sidoides group. However, differences in other components of the BSS (dyspnoea, chest pain, and crackle) before and after medication were not statistically significant (Fig. 3, Supplementary Table 1). Patients treated with DW1601 showed a higher response rate than those treated with DW16011 (78.8% vs. 60.3%, p = 0.02) or P. sidoides (78.8% vs. 53.1%, p = 0.002) at day 4. In addition, the response rates at day 7 were 100% for the DW1601 group, 85.7% for the DW16011 group, and 85.9% for the P. sidoides group (Fig. 4, Supplementary Table 2). DW1601-treated patients reported a significantly higher satisfaction rate than DW16011- or P. sidoides-treated patients both on day 4 and day 7 (Supplementary Table 3).
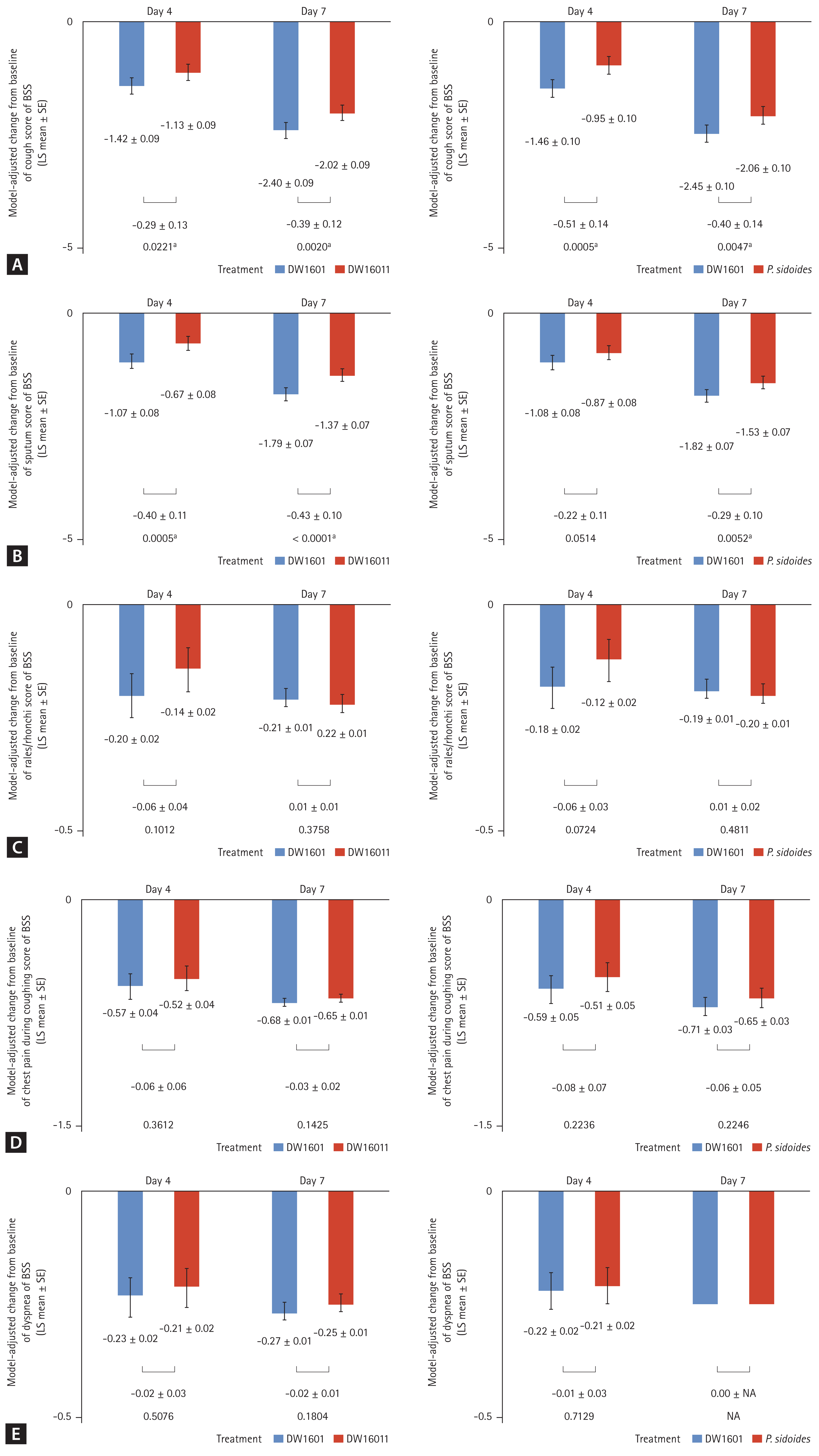
Comparison of changes from baseline in (A) cough score, (B) sputum score, (C) rales/rhonchi score, (D) chest pain score, and (E) dyspnea score between DW 1601 and control (DW 16011 or Pelargonium sidoides) after 4 and 7 days of medication. BSS, bronchitis severity score; LS, least square; SE, standard error. ap < 0.05.
Safety
Totals of five patients (7.5%) in the DW1601 group, six patients (8.6%) in the DW16011 group, and six patients (9.2%) in the P. sidoides group reported treatment-emergent adverse events. In addition, four patients (6.0%) in the DW1601 group, six patients (8.6%) in the DW16011 group, and four patients (6.2%) in the P. sidoides group reported adverse drug reactions. The reported adverse events were somnolence, nausea, facial oedema, and dry mouth, and all resolved spontaneously. There were no severe adverse events, such as death, near-death, irreversible functional disability, or an aggravated condition requiring hospital admission (Supplementary Table 4).
DISCUSSION
Acute bronchitis is a common disease in the outpatient clinical setting and is caused by viral infection or some bacterial infections [1–4,22]. Although antibiotics are generally not indicated for the majority of patients with acute bronchitis, clinicians might prescribe antibiotics to relieve symptoms if cough and sputum are persistent. In addition, persistent cough could lead to dysphonia, social isolation, and decrement in quality of life [8,9,22,23]. To decrease antibiotic overuse and harmful consequences, cough and sputum production need to be controlled. Many drugs have been developed to control cough and sputum in patients with acute bronchitis. In general, patients with acute bronchitis are treated with symptomatic agents (e.g., antitussives, expectorants, mucolytics, and bronchodilators), and oral pill combinations of these drugs are preferred to improve medication compliance [5,6,11–13].
DW1601 is a new oral fixed dose combination syrup consisting of DW16011 (a dihydrocodeine compound) and P. sidoides, which could facilitate rapid recovery from acute bronchitis symptoms including cough and sputum production. The primary purpose of this study was to test the hypothesis that DW1601 is superior to DW16011 or P. sidoides in reducing BSS on day 4, and our study result clearly proved this hypothesis through statistically significant differences among experimental groups. In evaluating the effectiveness of expectorants and antitussives, the BSS consisting of five components of cough, phlegm production, rales/rhonchi, chest pain during coughing, and dyspnoea assessed by physicians is considered a valid measurement tool; the change of total BSS is important [20,21]. The study showed superiority of DW1601 in reducing total BSS not only at day 4, but also at day 7. Individual cough or sputum component BSS was also superior in the DW1601 group at days 4 and 7. Moreover, participants treated with DW1601 showed higher rates of response and satisfaction than those of the control group. These results clearly demonstrated that DW1601 is an effective drug for acute bronchitis by rapidly relieving symptoms and prolonging therapeutic duration.
Recently, other studies have shown that Pelargonium extract could be effective in improving cold symptoms in patients with human coronavirus infection [24–26]. Considering that DW1601 contains P. sidoides, DW1601 might be effective in reducing cough and sputum production in patients with human coronavirus infection [24–26]. This is important in the COVID-19 pandemic period. If DW1601 is effective in controlling symptoms in patients with COVID-19, this should improve patient satisfaction and medication compliance. This would relieve the social stigma currently experienced by individuals with persistent of cough and sputum production. Further research on this issue is needed.
Oral fixed dose combination syrup could have more side effects than single drugs. However, this study showed that the proportion of side effects in the DW1601 group was similar to those in the DW16011 group and P. sidoides group. In the DW1601 group, the adverse events were somnolence, nausea, facial oedema, and dry mouth; these resolved spontaneously. There were no severe adverse events such as death, near-death, irreversible functional disability, or an aggravated condition that required admission. Therefore, DW1601 is a relatively safe drug.
This study had several limitations. First, we used the BSS, a subjective scale, as an indicator to evaluate clinical outcomes. However, the BSS scale has been validated in patients with acute bronchitis [20,21]. Second, our trials were conducted on relatively young people and women. Given that the proportion of elderly patients among outpatients is rapidly increasing due to the aging of the global population [27,28], additional studies are needed to evaluate the efficacy and safety of DW1601 in elderly patients. Finally, the number of patients in our trial was relatively small. However, the number was sufficient to evaluate the efficacy and safety of DW1601 compared to DW16011 or P. sidoides in patients with acute bronchitis.
In conclusion, DW1601 was superior to DW16011 or P. sidoides in reducing total BSS in patients with acute bronchitis at 4 days and at 7 days after medication. In addition, compared to DW16011 or P. sidoides, DW1601 showed superior patient satisfaction due to reduction of symptoms and was a relatively safe drug with few side effects. Additional studies with various acute bronchitis patient cohorts are warranted to validate our findings.
KEY MESSAGE
1. DW1601 was superior to DW16011 or Pelargonium sidoides in improving symptoms of acute bronchitis at 4 day and 7 days after medication..
2. DW1601 showed a higher response rate for symptoms and patient satisfaction with symptom reduction than DW16011 or P. sidoides.
3. DW1601 was a relatively safe drug with few side effects.
Notes
This trial was supported by Daewon Pharmaceutical company, Republic of Korea. This sponsor provided support for laboratory tests, transportation cost of patients, ECG/Chest X-ray, and investigational products.





