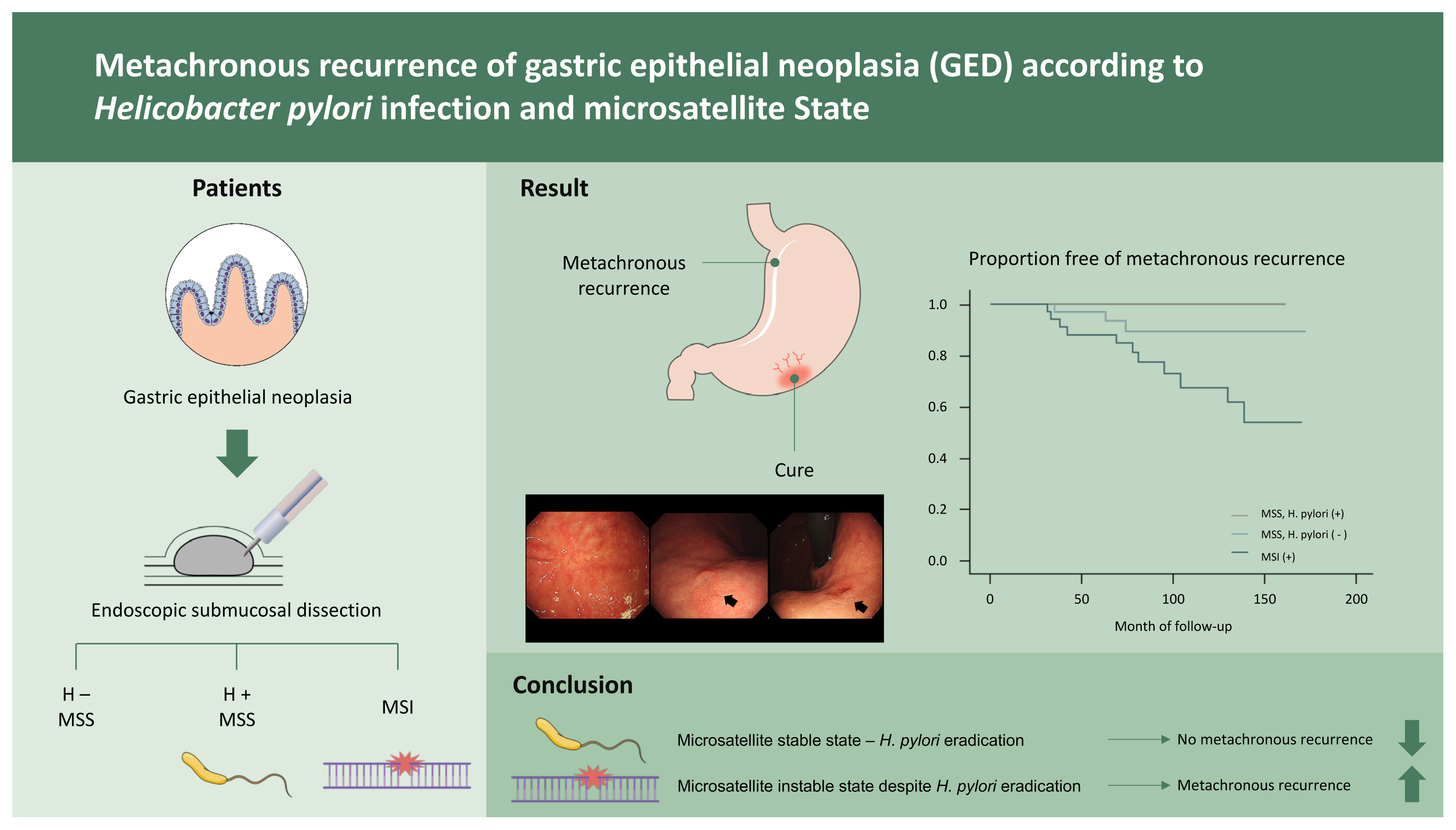
Clinical outcomes of metachronous recurrence of gastric epithelial neoplasia based on Helicobacter pylori infection status and microsatellite stability
Article information
Abstract
Background/Aims
Helicobacter pylori eradication may prevent the recurrence of gastric epithelial neoplasia after endoscopic treatment. However, H. pylori eradication therapy is unlikely to prevent gastric cancer. This study determined the long-term results and clinical outcomes of patients with gastric epithelial neoplasia based on H. pylori infection status and microsatellite stability (MSS).
Methods
Patients diagnosed with gastric epithelial neoplasia who underwent an endoscopic mucosal resection or submucosal dissection between 2004 and 2010 were included in this retrospective study. During the follow-up period (range, 4 to 14 years), disease recurrence was monitored, and tissue examinations were conducted for seven sets of microsatellite loci initially linked to the tumour suppressor gene locus. When H. pylori infection was identified, patients underwent eradication therapy.
Results
The patients (n = 120) were divided into three groups: H. pylori-negative with MSS, H. pylori-positive with MSS, and microsatellite instability (MSI). After H. pylori eradication, the rate of metachronous recurrence was significantly different in the MSI (28.2%) and MSS groups (3.7%, p < 0.01). The mean duration of recurrence was 77 months (range, 24 to 139) in the MSI group. There was no recurrence after eradication therapy in patients who were positive for H. pylori in the MSS group.
Conclusion
H. pylori eradication could help prevent gastric cancer recurrence in patients with stable microsatellite loci. Careful, long-term monitoring is required in patients with unstable microsatellite loci.
INTRODUCTION
Several epidemiologic studies have suggested that Helicobacter pylori eradication has prophylactic effects against gastric cancer [1,2]. Randomized controlled trials have revealed that H. pylori eradication therapy significantly reduces the incidence of metachronous gastric cancer after endoscopic resection and reverses carcinogenesis events as compared to states of persistent infection [3,4]. However, the effects of H. pylori eradication therapy in high-risk populations are less clear [5]. The occurrence of gastric cancer after H. pylori eradication (termed H. pylori negative gastric cancer) is increasing. In a prospective trial, the eradication of H. pylori did not significantly reduce the incidence of metachronous gastric carcinoma during a 3-year follow-up period, and there were insufficient long-term data to determine the long-term efficacy of H. pylori eradication [6]. H. pylori eradication alone does not prevent the development of cancer from gastric dysplasia. A recent meta-analysis suggested that H. pylori eradication may reduce metachronous recurrence for a minimum of 5 years, though recurrence may not be reduced during the first 5 years after eradication [7]. However, a significant number of patients had metachronous recurrence despite H. pylori eradication therapy. Therefore, prospective studies reporting well-controlled and long-term outcomes are necessary.
The effects of microsatellite instability (MSI) on gastric carcinogenesis were examined alongside those of H. pylori eradication in this study. Four genomic tumour subgroups of primary gastric adenocarcinomas have been identified, including an association with Epstein-Barr virus infection, MSI, chromosomal instability, and genomically-stable tumours [8]. MSI-positive gastric cancer is associated with the presence of H. pylori [9] and has several identifiable clinical characteristics. The mismatch repair genes mutL homolog 1 (MLH1) and mutS homolog 2 (MSH2) are involved in the disease. MSI-positive gastric cancers are more prevalent in older patients, females, and those with antral cancer, intestinal-type cancer, or a favourable prognosis [10,11].
This study determined the long-term results and clinical outcomes of patients with gastric epithelial dysplasia or early gastric cancer who underwent endoscopic mucosal resection or submucosal dissection based on their H. pylori infection status and microsatellite stability (MSS).
METHODS
Patients with gastric epithelial dysplasia or early gastric cancer who underwent endoscopic mucosal resection or submucosal dissection from 2004 to 2010 were included in this study. Gastric epithelial dysplasia and early gastric cancer were classified according to the revised Vienna Classification System [12]. The patients were recruited based on a previous study [13]. This study was approved by the Institutional Research Ethics Board of the Catholic University of Korea (VC20RASI0196) and conforms to the principles of the Declaration of Helsinki. All patients provided informed consent.
Surveillance esophagogastroduodenoscopy was performed after endoscopic submucosal dissection using GIF-H260, GIF-HQ260, and GIF-XQ290 gastroscopes (Olympus Medical Inc., Tokyo, Japan) at 6 and 12 months of follow-up in patients with category III tumours. Annual examinations were conducted for 5 years, and a 2-year follow-up protocol was recommended. H. pylori-positive patients were identified during each endoscopic examination using silver staining or rapid urease test. Surveillance esophagogastroduodenoscopy was performed at 6, 12, 18, and 24 months of follow-up in patients with category IV or V tumours. Thereafter, examinations were conducted annually for 3 years, and a 2-year follow-up protocol was recommended. This scheduled endoscopic program was paid for by the Korea National Insurance system. Patients infected with H. pylori were immediately administered eradication therapy and were successfully treated after one or two cycles of eradication therapy.
The primary outcome in this study was recurrence, which was categorised as marginal, metachronous, or synchronous. The secondary outcomes were clinical outcomes such as subsequent endoscopic procedures or surgical interventions. A synchronous lesion was defined as a concomitant gastric epithelial lesion identified at the time of endoscopic resection or a lesion detected within 12 months postoperatively. A metachronous lesion was defined as a lesion diagnosed more than 12 months postoperatively at the site of the primary lesion or located in a different part of the stomach (Fig. 1) [14,15].
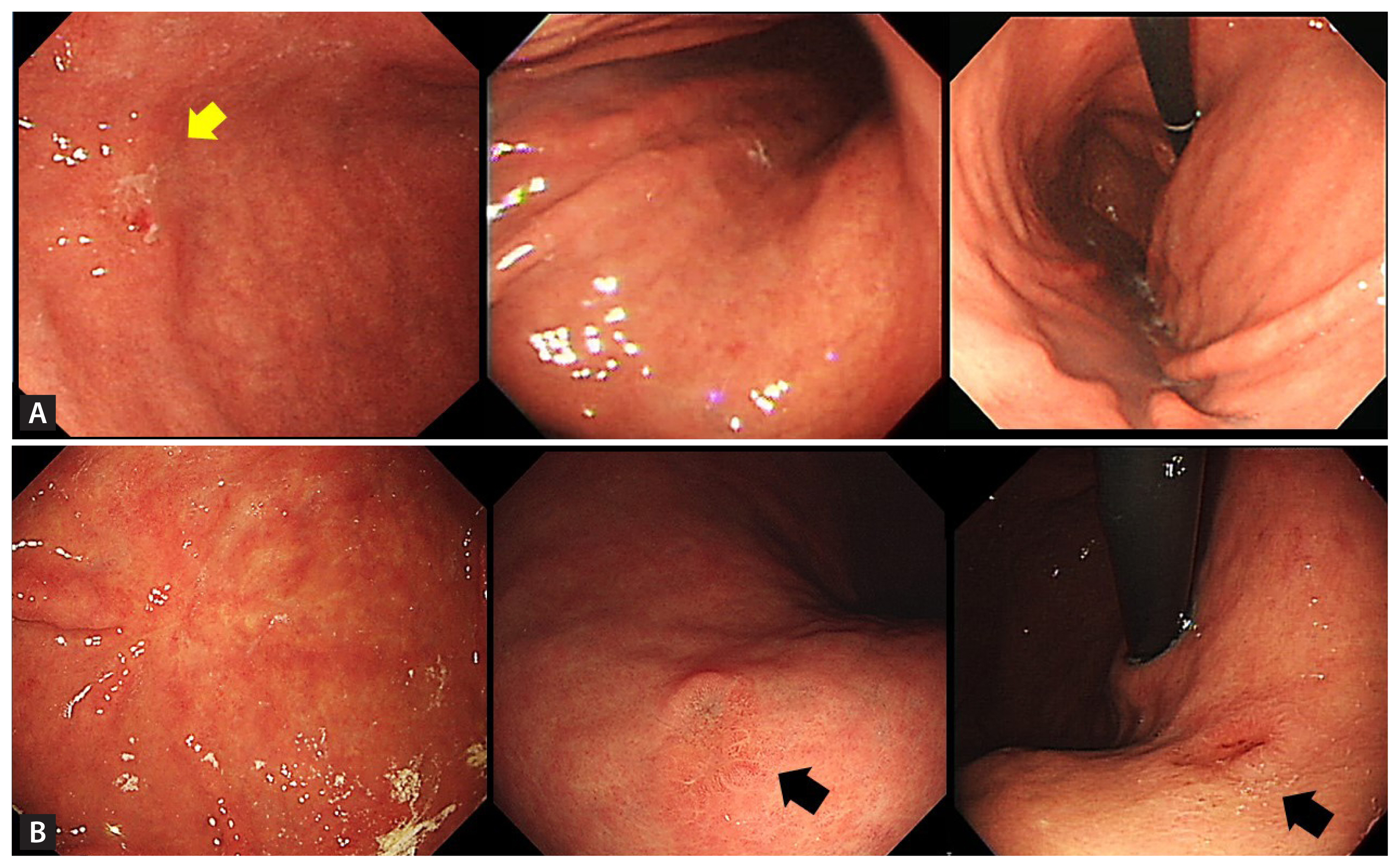
(A) A 1.6-cm elevated mucosal lesion with a central depression (yellow arrow) is identified on the anterior wall of the proximal antrum on initial endoscopy. (B) A surveillance endoscopy 95 months after endoscopic submucosal dissection shows a surgical scar on the anterior wall of the proximal antrum. A 1.5-cm elevated mucosal lesion with central depression is shown on the posterior wall of the high body (black arrows). It is classified as a metachronous lesion.
DNA extraction
Two histopathologists confirmed the diagnoses with tissue samples obtained during endoscopic therapy. The tissue samples were excluded from the study if the histopathologists did not agree on a diagnosis. All normal tissues had grossly intact mucosa, were at least one centimetre from the mucosal lesion, were obtained by gastric biopsy immediately after endoscopic mucosal resection, and had no microscopic evidence of malignant cells. Two 40 μm-thick tissue sections from tumour and normal tissues were placed on glass slides, dehydrated using graded ethanol solutions, and dried without a cover glass. The DNA was extracted from the tissues during an overnight incubation using 20 μL of extraction buffer (100 mmol/L Tris-HCl; 2 mmol/L ethylene diamine tetraacetic acid [EDTA], pH 8.0; and 400 μg/mL of proteinase K) at 55°C. The samples were boiled for 7 minutes to inactivate the proteinase K, and 1 μL of each sample was used in each polymerase chain reaction (PCR) amplification.
PCR-single-stranded conformation polymorphism analysis
PCR-single-strand conformation polymorphism was used to assess the MSS. Seven loci that are associated with tumour suppressor genes were analysed. Using PCR, the DNA was amplified at the microsatellite loci linked to the adenomatous polyposis coli (APC) locus on 5q21 (D5S505), possible tumour suppressor or senescence gene loci on 10p15 (D10S501 and D10S602), p53 locus on 17p13 (TP53), breast cancer type 1 (BRCA1) locus on 17q21 (D17S855), and deleted in colorectal cancer (DCC) loci on 18q21 (D18S58 and D18S61).
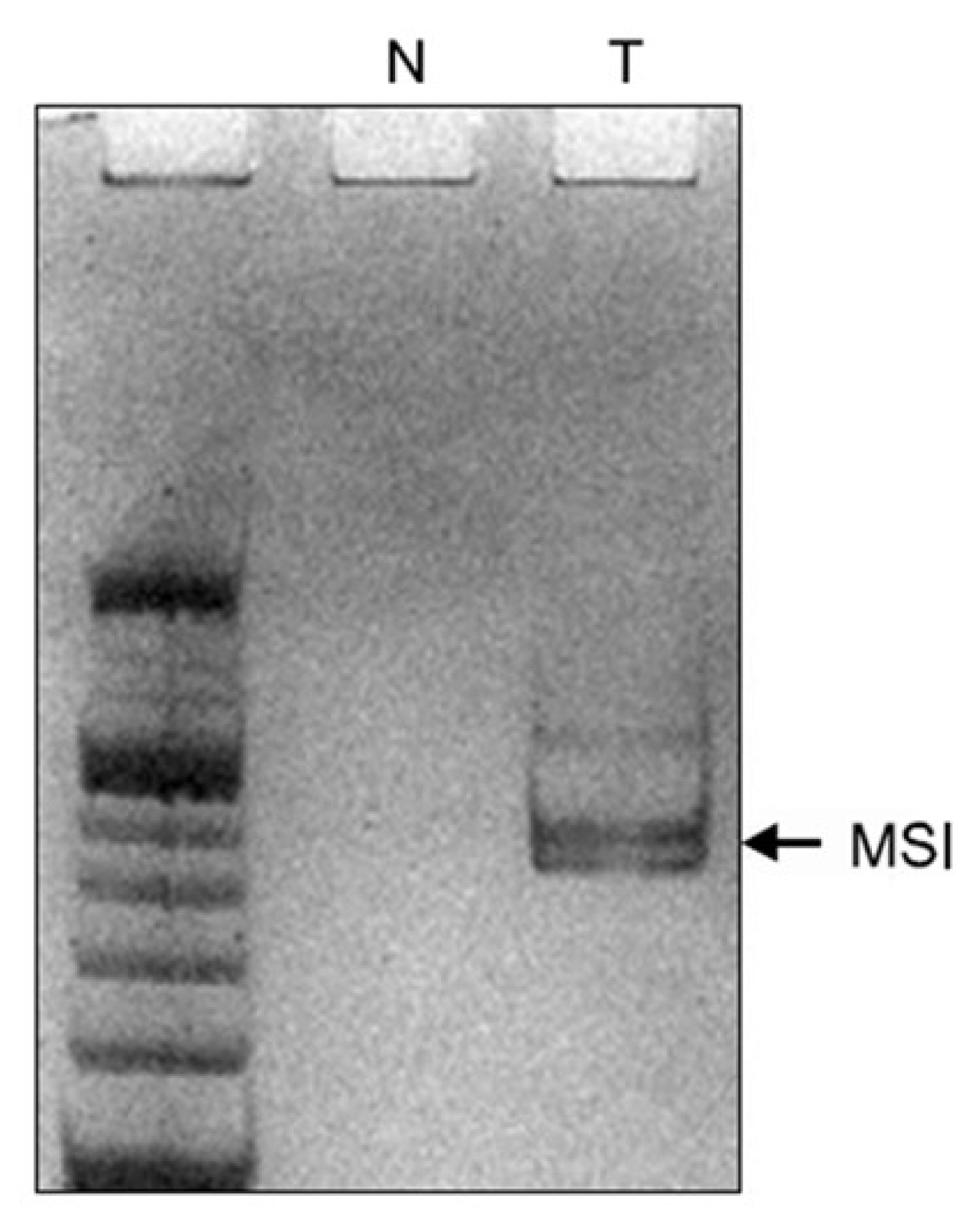
A 10% neutral polyacrylamide gel electrophoresis was performed as previously described. In brief, the gel consisted of 4 mL 30% acrylamide (29:1) solution, 2.4 mL 5 × Tris/borate/ethylenediaminetetraacetic acid (TBE) solution, 5.6 mL ddH2O, 200 μL 10% ammonium persulfate, and 10 μL Thermo Scientific Pierce Tetramethylethylenediamine (TEMED). PCR product (10 μL) and loading buffer (2 μL; 95% formamide, 10 mM NaOH, 0.1% bromophenol blue, and 0.1% xylene cyanole) were combined, centrifuged for 15 seconds, denatured at 93°C for 3 minutes, cooled in ice for 10 minutes, and loaded on the gel. Electrophoresis was conducted in 0.5 × TBE buffer for 2 hours at 100 V and at room temperature. MSI was defined as additional bands in the tumour sample that were not observed in the corresponding normal sample or as a band shift in the tumour sample that contrasted with the corresponding normal bands (Fig. 2).
Based on the DNA analyses, patients were divided into three groups: H. pylori negative with MSS; H. pylori positive with MSS; and MSI regardless of H. pylori infection status. The same follow-up protocol was used for all three groups.
Statistical analysis
Continuous variables are presented as XX, and categorical variables are presented as XX. The Student’s t test was used to compare continuous variables between the two groups. Differences between categorical variables were evaluated using the chi-square test. All statistical analyses were conducted using SPSS software version 25.0 (IBM Co., Armonk, NY, USA). Statistical significance was set at p < 0.05.
RESULTS
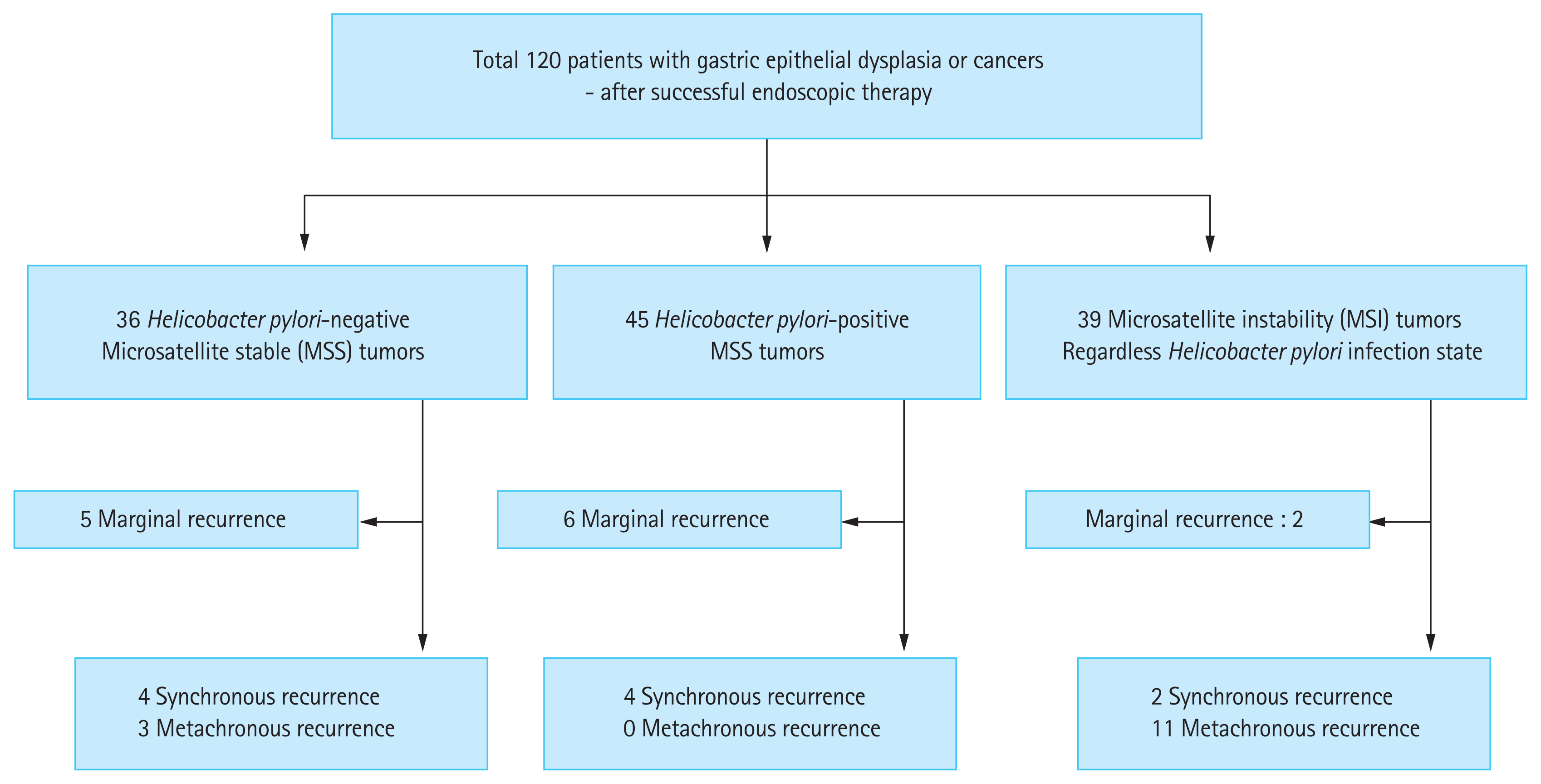
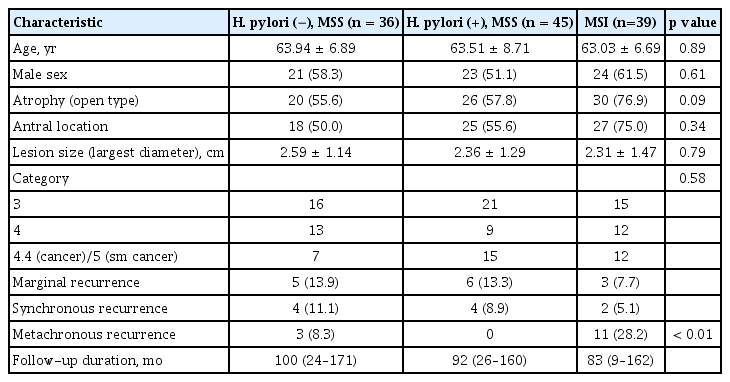
A total of 120 patients (68 males) who underwent eradication therapy within 1 year of endoscopic submucosal dissection were included in this study. No patients had re-infection cases during the follow-up period. Thirty-six patients were H. pylori negative with MSS, 45 were H. pylori positive with MSS, and 39 had MSI (including 25 [64.1%] with H. pylori). The patients’ clinicopathological characteristics are shown in Fig. 3. There were no differences in age, sex, presence of open type atrophy, lesion size, frequency of antral location, or subgroup of epithelial dysplasia between the groups (Table 1). The mean follow-up period was 90.75 ± 43.65 months.
Recurrent lesions were observed in 38 (31.7%) patients. The marginal recurrence rate of primary lesions was 11.7% (14/120). Excluding marginal recurrence, the true recurrence rate was 20.0% (24/120). Three patients (8.3%) who were H. pylori negative with MSS had metachronous recurrence, and no patients who were H. pylori positive with MSS had metachronous recurrence. Eleven (28.2%) patients with MSI had metachronous recurrence (Table 2). The recurrence (synchronous and metachronous) was significantly higher in the MSI group than in the MSS group (33.3% [13/39] vs. 13.6% [11/81], respectively; p = 0.01). Metachronous recurrence was also significantly higher in patients with MSI compared to those with MSS (28.2% [11/39] vs. 3.7% [3/81], respectively; p < 0.01) (Fig. 4). The mean durations of recurrence were 77 months (range, 24 to 139) in the MSI group and 58 months (range, 35 to 74) in the MSS group. A total of 70 patients underwent H. pylori eradication therapy, and six (8.6%) patients with MSI had metachronous recurrence. No metachronous recurrence was observed in patients with H. pylori infection and MSS after eradication therapy.

Kaplan-Meier curve for metachronous free survival. H. pylori, Helicobacter pylori; MSS, microsatellite stability; MSI, microsatellite instability.
Most patients with recurrence underwent repeated endoscopic therapy. Complete resection was conducted in 28 (73.7%) patients. Five patients with MSI were surgically treated due to incomplete resections (n = 4) or not meeting the criteria for a curative resection (n = 1). In the MSS group, four patients underwent operations and one had metastasis. The requirement of surgical resection was not statistically different between patients with MSS and those with MSI (p = 0.27).
DISCUSSION
A multistep, adenoma-to-carcinoma sequence has been established for colon cancer [16]. The multi-step carcinogenic cascade model of H. pylori-induced intestinal-type gastric adenocarcinoma includes the change from normal gastric epithelium to chronic gastritis to chronic atrophic gastritis to intestinal metaplasia (IM) to epithelial dysplasia to gastric cancer [17]. H. pylori eradication is strongly correlated with an improvement in atrophic gastritis in the corpus and antrum of the stomach and in the antrum but not in the corpus in patients with IM [18]. It is unclear if patients with dysplasia benefit from H. pylori eradication, though it is believed to be unlikely.
The risk of metachronous recurrence after endoscopic submucosal dissection is higher in patients with persistent H. pylori infection than in those whose infection has been eradicated [19–22]. H. pylori eradiation may reduce the risk of metachronous recurrence following endoscopic therapy. However, in some patients, H. pylori eradication therapy does not eliminate the risk of gastric cancer [23,24]. In a large-scale, prospective, randomised controlled trial, 4.1% of patients in whom H. pylori had been eradicated had metachronous cancer recurrence during a 5.9-year follow-up [9]. In this study, 70 patients underwent H. pylori eradication therapy and 8.6% had metachronous recurrence with a median follow-up period of 7.0 years. A recent meta-analysis revealed that metachronous gastric lesions developed in 6.0% of patients who underwent eradication therapy compared with 7.2% of patients with persistent H. pylori and 5.9% of patients without H. pylori.
Limited data regarding the risk factors of metachronous recurrence have been reported, though family history of gastric cancer and relatively old age (> 70 years) have been indicated as risk factors [25,26]. As older age is a risk factor for most cancers, this risk factor is well-established. However, Lim et al. [27] reported that the absence of H. pylori infection was an independent risk factor for metachronous neoplasms, including dysplasia. This may be due to the fact that long-term H. pylori infection can lead to severe atrophic gastritis with IM, rendering H. pylori undetectable. Hypochlorhydria or achlorhydria following severe atrophy results in the overgrowth of bacteria in the gastric microbiota, leading to gastric cancer. A previous study reported that the risk for gastric cancer according to 5-year incidence rates was 9.8% in patients with corpus IM and 10.0% in patients with severe atrophy [28]. In addition, the genetic events induced by H. pylori infection may be irreversible or on-going after H. pylori eradication [29]. H. pylori infection may lead to gastric cancer via gene mutations that cannot be reversed with eradication therapy. This hypothesis may explain why the reduction of metachronous gastric cancer via H. pylori eradication is ambiguous in high-risk populations. Several researchers have attempted to reverse gastric carcinogenesis via H. pylori eradication with no success [30,31]. As a surrogate marker for gastric carcinogenesis, MSI may be used as a marker for H. pylori-negative gastric cancer after eradication therapy. While the Bethesda Panel may be correlated with MSI, especially in colorectal cancers [32], the gene loci identified in this study may better reflect MSI in patients with gastric cancer. More studies are required to elucidate the exact loci in patients with gastric cancer.
This study is not without limitations, including the retrospective design. However, the findings remain significant as the endoscopic follow-up program was standardized for all patients after endoscopic submucosal dissection. In addition, the overall prevalence of H. pylori infection was much lower than that expected in an Asian cohort with gastric cancer, as patients with a history of eradication were not excluded due to lack of documentation of previous eradication therapy. H. pylori infection was diagnosed using silver staining and rapid urease tests. More sensitive methods, such as serological testing or molecular detection using PCR, should be used in future studies. To overcome this limitation, tissue-based H. pylori tests were repeated during each endoscopic examination. Although the eradication history prior to endoscopic submucosal dissection was unknown, it is likely that most patients did not previously undergo H. pylori eradication therapy, as medical insurance only covers H. pylori eradication therapy after an endoscopic submucosal dissection or in patients with peptic ulcer disease in Korea. Another limitation of this study is that the validity of the MSI is unclear, and repeated evaluations of MSI are lacking. Five microsatellite markers (BAT25, BAT26, D2S123, D5S346, and D17S250) were identified for colon cancer in the National Cancer Institute Workshop on MSI [32]. However, no consensus regarding MSI in patients with gastric cancer has been reached, especially during the recruitment period of this study. Various microsatellite markers can be used to measure MSI. A recent study suggested that MSI in mismatch repair and tumour suppressor genes and gene expression profiling can provide important targets for the development of biomarkers in gastric cancer [33]. Several molecular markers may be used as MSI biomarkers for the diagnosis of gastric tumorigenesis after further validation. The effectiveness and cost-effectiveness of biomarkers must be determined. The current cost of examination for five MSI markers is approximately 500 USD when not covered by medical insurance. This study is also limited by the fact that patients’ family history of gastric cancer was not clearly documented. This risk factor may help explain the high prevalence of H. pylori-negative patients in this study. Future studies should include a thorough family history.
In conclusion, H. pylori eradication therapy may be less effective in preventing metachronous recurrence in patients with MSI. When MSI was detected in gastric lesions, metachronous recurrences occurred over a relatively long follow-up period. These results indicate that MSI affects the development of epithelial dysplasia consistently and chronically. Therefore, careful long-term surveillance is necessary. However, the maximal benefit of preventing gastric cancer can be achieved by H. pylori eradication therapy in patients with MSS. The identification of patients at high risk for gastric cancer after H. pylori eradication is necessary, and MSI may be a strong predictive marker.
KEY MESSAGE
1. Helicobacter pylori eradiation may reduce the risk of metachronous recurrence of gastric cancer following endoscopic therapy. However, in some patients, H. pylori eradication therapy does not eliminate the risk of gastric cancer.
2. Microsatellite instability may be used as a marker for H. pylori-negative gastric cancer after eradication therapy.
3. H. pylori eradication therapy in patients with microsatellite stability could give a great benefit of preventing gastric cancer.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.