Benefits of lumican on human bone health: clinical evidence using bone marrow aspirates
Article information
Abstract
Background/Aims
Lumican, a small leucine-rich proteoglycan, has shown osteoprotective effects by synchronously stimulating bone formation and suppressing bone resorption. To clarify the role of lumican in human bone metabolism, the association between lumican concentrations and osteoporosis-related phenotypes was evaluated using bone marrow (BM) samples directly reflecting local microenvironments.
Methods
BM aspirates were obtained from 77 patients during hip surgery for either fragility hip fractures (HF) (n = 29) or osteoarthritis (n = 48) and centrifuged. Concentrations of lumican and biochemical bone markers in BM supernatants were measured using enzyme linked immunosorbent assays.
Results
After considering confounders, lumican concentrations in BM supernatants were 16.9% lower in patients with HF than in controls, with each increase in the standard deviation of lumican concentration being associated with a 61% lower likelihood of HF. The odds ratios for HF decreased linearly with increasing lumican tertiles in BM, with the odds of having fragility HF markedly lower in participants in the highest than in the lowest lumican tertile. Higher lumican level correlated significantly with higher femur neck bone mineral density and higher bone-specific alkaline phosphatase levels, but not with tartrate-resistant acid phosphatase 5b concentrations, in BM supernatants.
Conclusions
These data clinically validate previous in vitro and animal experiments showing the beneficial roles of lumican for bone homeostasis and suggest that lumican may contribute to a reduction in fracture risk in humans mainly through its stimulation of bone formation.
INTRODUCTION
Osteoporotic fracture (OF) substantially increases disability, morbidity, and mortality in older adults [1,2]. As the worldwide population ages, so do the frequency and importance of osteoporosis and related fractures, which are associated with large socioeconomic burdens [3]. At present, anti-resorptive agents, such as bisphosphonates and denosumab, are the most widely used medications worldwide in the treatment of osteoporosis [4]. Due to a coupling phenomenon, however, these agents concomitantly suppress bone formation, limiting their efficacy and possibly leading to increases in adverse events [5–7]. Although bone-forming agents, such as intermittent parathyroid hormone and romosozumab, have been suggested as alternatives, their anabolic activities last for only 1 or 2 years [8,9], which may not be sufficient to effectively prevent OF over a long period. Consequently, efforts are ongoing to identify novel therapeutic targets for osteoporosis and to maintain skeletal health in aging populations.
Lumican, a member of the small leucine-rich proteoglycan (SLRP) family originally identified as a main keratan-sulfate-containing proteoglycan of the cornea [10], is ubiquitously expressed in a variety of tissues and participates in key biological activities, such as cell migration, adhesion, proliferation, and differentiation [11,12]. Not surprisingly, lumican has important roles in bone metabolism by suppressing osteoclast differentiation and in vitro bone resorption as well as increasing osteoblastogenesis and calvaria bone formation in mice [13,14]. However, despite the involvement of lumican in bone metabolism on the cellular level and in animals, the relationship of lumican to osteoporosis-related phenotypes and their underlying mechanisms in humans have not yet been investigated. This study, using bone marrow (BM) samples directly reflecting the bone microenvironment, evaluated whether lumican concentration was significantly associated with hip fracture (HF) status and assessed whether lumican concentration was associated with bone mass and biochemical bone markers in the elderly.
METHODS
Study participants
This cross-sectional study enrolled consecutive patients aged ≥ 65 years who underwent hip surgery due to either fragility HF or osteoarthritis at the Department of Orthopaedics of Asan Medical Center (AMC), Seoul, Korea, between March 2013 and July 2014. Fragility HFs are defined as fractures caused by mechanical forces that would not ordinarily result in fracture, a condition known as low-energy trauma [3]. Subjects who had taken drugs possibly affecting bone metabolism for more than 6 months or within the 12 months prior to hip surgery, such as hormone replacement therapy, systemic glucocorticoids, or bisphosphonates, were excluded. Subjects with diseases that might lead to secondary osteoporosis, such as rheumatoid arthritis or thyroid disorders, were also excluded. To eliminate the effects of systemic illness, subjects were also excluded if they had fever (oral temperature ≥ 38.0°C), abnormal findings on complete blood counts of leukocytes (< 4.0 or > 10.0 × 109/L) or platelets (< 150 or > 350 × 109/L), or abnormal kidney or liver functions. Finally, subjects with fractures not resulting from low-energy trauma, such as motor vehicle accidents or falls above a standing height, were excluded. A total of 122 eligible subjects did not meet exclusion criteria. After receiving their informed consent, BM samples were collected during hip surgery from 77 of these subjects, 29 from patients who underwent hip surgery for fragility HF and 48 from patients who underwent hip surgery for osteoarthritis.
Patients were administered questionnaires to determine history of medication use, previous medical or surgical procedures, alcohol intake (≥ 3 U/day), smoking (current smoker), and reproductive status (including pre- and post-menopausal). The study protocol conformed to the Ethical Principles for Medical Research Involving Human Subjects, as defined by the Declaration of Helsinki, and was approved by the AMC Institutional Review Board (2012-0406). All enrolled participants provided written informed consent.
Biochemical measurements in BM samples
BM samples were obtained from all patients during hip surgery, which was performed within 4 days after onset of HF in most patients. The samples were centrifuged at 3,000 rpm for 5 minutes at 4°C, and the supernatants were carefully decanted to exclude cell components. All samples showing evidence of clotting or hemolysis were discarded. Lumican concentrations in BM supernatants were measured with Solid Phase Sandwich enzyme-linked immunosorbent assay (ELISA) kits (Cat# DY2846–05, R&D Systems, Minneapolis, MN, USA; lower limit of detection, 0.125 ng/mL), according to the manufacturer’s instructions. Other bone biochemical markers measured with immunoassay kits in BM supernatant samples included receptor activator of nuclear factor-κB ligand (RANKL; Cat# K1016, Immunodiagnostic Systems, Scottsdale, AZ, USA), osteoprotegerin (OPG; Cat# ab100617, Abcam, Cambridge, MA, USA), bone-specific alkaline phosphatase (BSALP; Cat# MBS262250, Mybiosource, San Diego, CA, USA), and tartrate-resistant acid phosphatase 5b (TRAP-5b; Cat# SB-TR201A, Immunodiagnostic Systems). The inter- and intra-assay coefficients of variation for each assay were < 9.3% and < 3.5%, respectively, for RANKL; < 12% and < 10%, respectively, for OPG; < 12% and < 7%, respectively, for BSALP; and < 9% and < 9%, respectively, for TRAP-5b.
Bone mineral density assessment
Bone mineral density (BMD; g/cm2) at the lumbar spine (L1–L4), femur neck, and total femur were measured by dual-energy X-ray absorptiometry with a Lunar densitometer (Prodigy, Madison, WI, USA). This instrument had precisions of 0.67% for the lumbar spine and 1.25% for the femur neck, as determined by measuring BMD in 17 volunteers who were not enrolled in this study. These volunteers were required to individually undergo five scans on the same day and to climb on and off the table between scans. BMD was measured during hospitalization for hip surgery or at the first outpatient visit after surgery. Therefore, the time difference between BMD measurement and the acquisition of BM aspirates would not exceed 1 month.
Statistical analysis
The baseline characteristics of the study participants with and without fragility HF were compared using chi-square tests for categorical variables and Student’s t tests for continuous variables. The multivariable-adjusted least-square mean (95% confidence interval [CI]) of lumican concentrations in BM supernatants in subjects with and without fragility HF were compared by analysis of covariance (ANCOVA). Potential confounders included sex, age, body mass index (BMI), alcohol drinking and smoking habits, and serum 25-hydroxyvitamin D3 (25-OH-D3), factors previously shown to be associated with human bone health. Multiple logistic regression analyses were performed to determine the odds ratios (ORs) for fragility HF according to lumican standard deviation (SD) increments and lumican tertiles in BM supernatants. Multiple linear regression analyses were performed to assess the associations of lumican concentrations with bone mass and biochemical bone markers in BM supernatants. Because of their skewed distributions, the concentrations of BSALP and TRAP-5b and the RANKL/OPG ratio were log transformed. All statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). A p < 0.05 was considered statistically significant.
RESULTS
The clinical characteristics of the 77 subjects who underwent hip surgery are described in Table 1. Of the 29 patients with fragility HF and the 48 with osteoarthritis, 22 (75.9%) and 33 (68.8%), respectively, were women. The mean ages of subjects with and without HF were 77.7 ± 9.5 years (range, 65 to 95) and 71.7 ± 5.6 years (range, 65 to 90), respectively. Weight, height, BMI, alcohol drinking and smoking habits, and serum 25-OH-D3 level did not differ significantly between these two groups. BMDs at all sites, including the lumbar spine, femur neck, and total femur, were significantly lower in patients who underwent hip surgery for fragility HF than for osteoarthritis (all p < 0.001). TRAP-5b concentrations in BM aspirates were significantly higher in participants with than without HF (p = 0.014). By contrast, the concentrations of BSALP, RANKL, and OPG, and the RANKL/OPG ratio in BM supernatants, did not differ significantly in participants with and without HF.

Clinical characteristics of the study participants who underwent hip surgery with or without fragility HF
Before and after adjustment for sex, age, and BMI, lumican concentrations in BM supernatants were 18.4% (p = 0.001) and 17.1% (p = 0.003) lower, respectively, in participants with than without HF (Fig. 1). These statistically significant differences persisted following additional adjustments for alcohol drinking and smoking habits and serum 25-OH-D3 concentrations (p = 0.004).
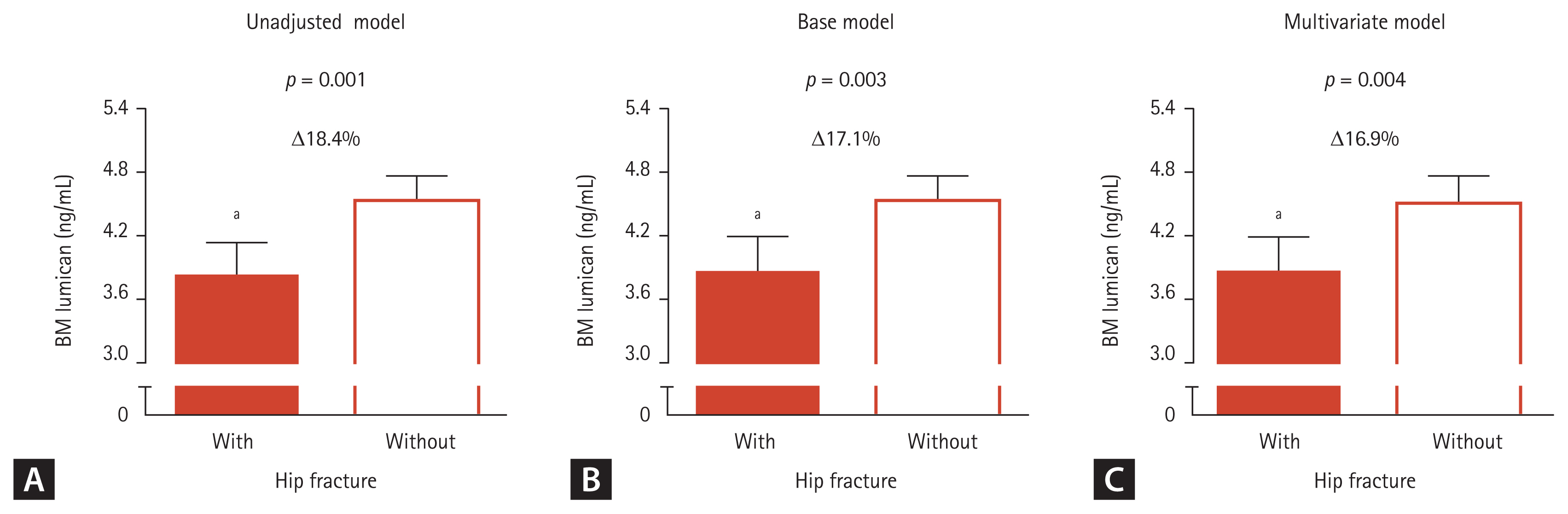
Lumican concentrations in bone marrow (BM) aspirates of patients with and without fragility hip fracture. After adjusting for confounders, the estimated means with 95% confidence intervals were generated and compared by analysis of covariance (ANCOVA). (A) Unadjustment model. (B) Base adjustment model: sex, age, and body mass index. (C) Multivariate adjustment model: sex, age, body mass index, alcohol and smoking habits, and serum 25-hydroxyvitamin D. Delta (Δ) indicates a difference between the two groups. ap < 0.05 compared with control group without hip fracture.
The ORs for fragility HF were assessed using logistic regression analyses. Regardless of adjustment models, each SD increase in lumican concentrations in BM supernatants was associated with a > 61% lower likelihood of HF (p = 0.002 to 0.007) (Table 2).
To determine whether the relationship of lumican concentration in BM supernatant with HF involves a threshold effect, participants were divided into three groups based on lumican concentrations in BM supernatants (Fig. 2). In crude, base, and multivariate adjustment models, the ORs for HF decreased in a linear fashion with increasing lumican tertiles (p for trend = 0.001 to 0.002), with the likelihood of fragility HF being markedly lower in participants in the highest (Q3) than the lowest (Q1) lumican tertile (p = 0.001 to 0.002).
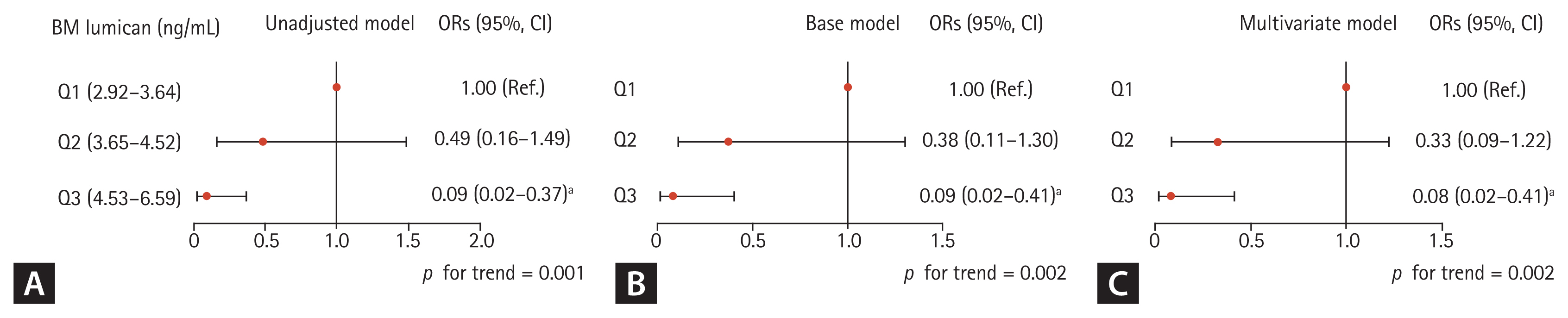
Odds ratios for fragility hip fracture according to lumican tertiles in bone marrow (BM) supernatant. (A) Unadjustment model. (B) Base adjustment model: sex, age, and body mass index. (C) Multivariate adjustment model: sex, age, body mass index, alcohol and smoking habits, and serum 25-hydroxyvitamin D. OR, odds ratio; CI, confidence interval. ap < 0.05 compared with the lowest tertile (Q1).
Linear regression analyses were performed to investigate the correlations of lumican concentration in BM supernatant with BMD and bone-related parameters in BM supernatants. After adjustment for sex (standardized regression coefficient β = −0.299), age (β = −0.228), BMI (β = 0.270), and other confounders, lumican concentrations in BM supernatants were positively and significantly associated with BMD of the femur neck (β = 0.231, p = 0.033, R-squared = 0.423) (Table 3), but not with BMD at the lumbar spine and total femur. In crude, base, and multivariate adjustment models, higher lumican concentration correlated significantly with higher BSALP concentration (p = 0.003 to 0.006), but not with TRAP-5b concentration or RANKL/OPG ratio, in BM supernatants (Table 4).
DISCUSSION
This study demonstrated that higher lumican concentrations in BM samples obtained from patients aged ≥ 65 years during hip surgery were significantly associated with a lower risk for fragility HF and a higher femur neck BMD after considering potential confounders. Interestingly, lumican concentration correlated positively with the concentration of BSALP, a marker of osteoblast differentiation, but not with the concentration of TRAP-5b or the RANKL/OPG ratio in BM aspirates. These findings provide the first clinical evidence that lumican may play beneficial roles in human bone metabolism, mainly through the stimulation of bone formation, thus contributing to the prevention of OF.
This study differs from previous studies in that it measured lumican and bone-related biochemical markers in BM aspirates. In general, most clinical studies utilize peripheral blood samples, as they are easier to acquire than most other types of biological fluids. However, systemic blood and local bone environments contain different types of cells, suggesting differences in their ability to metabolize various candidate factors. In fact, concentrations of RANKL, OPG, BASLP, and TRAP-5b in blood were found to be weakly or not at all associated with their concentrations in BM plasma [15]. To alleviate concerns that peripheral blood may not be a suitable medium for investigating the mechanisms underlying specific effectors in human bone, the role of lumican in human bone health was evaluated by collecting BM samples and preparing BM supernatants.
Molecules in the SLRP family have been classified into five subtypes, with each subtype being actively involved in various biologic functions, as well as being the main components of the extracellular matrix [16,17]. As with specific SLRPs, including epiphycan and biglycan, which are recognized as important modulators of bone homeostasis [18,19], lumican has been found to play roles in skeletal systems. In detail, lumican was shown to enhance bone formation by activating preosteoblast viability and differentiation, with these anabolic effects being mediated by integrin α2β1 receptor and extracellular signal-regulated kinase signaling in osteoblasts [13]. Lumican was also shown to suppress in vitro bone resorption and osteoclastogenesis by inhibiting the Akt pathway [14]. In contrast to these findings in cells and mice, however, the present clinical study found that lumican concentrations in BM aspirates were predominantly associated with a marker of bone formation, rather than a marker of bone resorption, in older adults. Despite findings consistently showing that lumican has osteoprotective effects, this discrepancy may be due in part to the notion that certain environments and processes in cells and animals differ from those in clinical studies and that the effects of candidate factors in vitro and in animals may not be fully applicable to humans.
Current anti-osteoporotic medications have limitations, including side effects and long-term ineffectiveness [4,5,7,9]. Efforts have therefore sought to identify novel drugs targeting osteoporosis. Previous experimental research demonstrated the dual actions of lumican with not only the increase of bone formation but the decrease of bone resorption [13,14]. Furthermore, the present study supported the possible roles of lumican in human bone metabolism, suggesting that this protein may be a candidate therapeutic factor reducing the risk of OF. Importantly, lumican is small in size, with a molecular weight of about 40 kDa [10], suggesting its advantageousness for drug development due to much easier administration and lower production costs. More extensive interventional studies, including evaluation of its pharmacokinetics and pharmacodynamics in animals and humans, are needed to evaluate the clinical applicability of lumican.
The present study had several limitations. First, it was cross-sectional study, precluding a determination of a causal relationship among variables. Although higher lumican concentrations in humans likely contribute directly to the maintenance of bone health based on previous experiments [13,14], there is the possibility that our results could have been generated due to a reverse causal relationship, which implies that the occurrence of fragility HF may have reduced lumican concentrations. Second, although BM samples are ideal for evaluating clinical mechanisms in relation to bone, BM fluid is difficult to collect, resulting in small sample sizes, which may limit their statistical power. Third, bone turnover is known to be increased after acute fracture, and thus these changes may have affected the present results. Finally, the study population consisted of patients who underwent hip surgery at a tertiary hospital, inevitably causing selection bias. Thus, our results may not be applicable to the general population.
In summary, this study found that higher lumican concentrations in BM supernatants correlated significantly with a lower likelihood of fragility HF, a higher bone mass, and a higher BSALP level in BM of elderly subjects, even after adjustment for confounders. These data clinically validate previous in vitro and animal experiments showing the protective effects of lumican on bone homeostasis and suggest that the positive association of lumican with osteoporosis-related parameters could be attributable to the increase in osteoblastogenesis in humans. Clinical studies evaluating the role of circulating lumican as a biomarker of osteoporosis and fracture may further clarify the importance of lumican to human bone health.
KEY MESSAGE
1. Higher lumican concentrations in bone marrow (BM) samples reflecting local microenvironments were significantly associated with a lower risk for fragility hip fracture and a higher bone mass at the femur neck.
2. Lumican concentration correlated positively with the concentration of a bone formation marker, but not with the concentration of resorption markers, in BM aspirates.
3. Lumican may play beneficial roles in human bone metabolism, mainly by stimulating bone formation, thus contributing to the prevention of osteoporotic fracture.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This study was supported by grants from the National Research Foundation of Korea (NRF) funded by the South Korean government (MSIT) (grant numbers: 2019R1A2C2006527 and 2021R1C1C2006842) and from the Asan Institute for Life Science, AMC, Seoul, South Korea (grant number: 2022IP0077).