The efficacy of EGFR-tyrosine kinase inhibitor in non-small cell lung cancer patients with synchronous brain metastasis: a real-world study
Article information
Abstract
Background/Aims
The optimal treatment (Tx) for epidermal growth factor receptor (EGFR)-mutant non-small cell lung cancer (NSCLC) patients with brain metastasis (BM) remains to be determined.
Methods
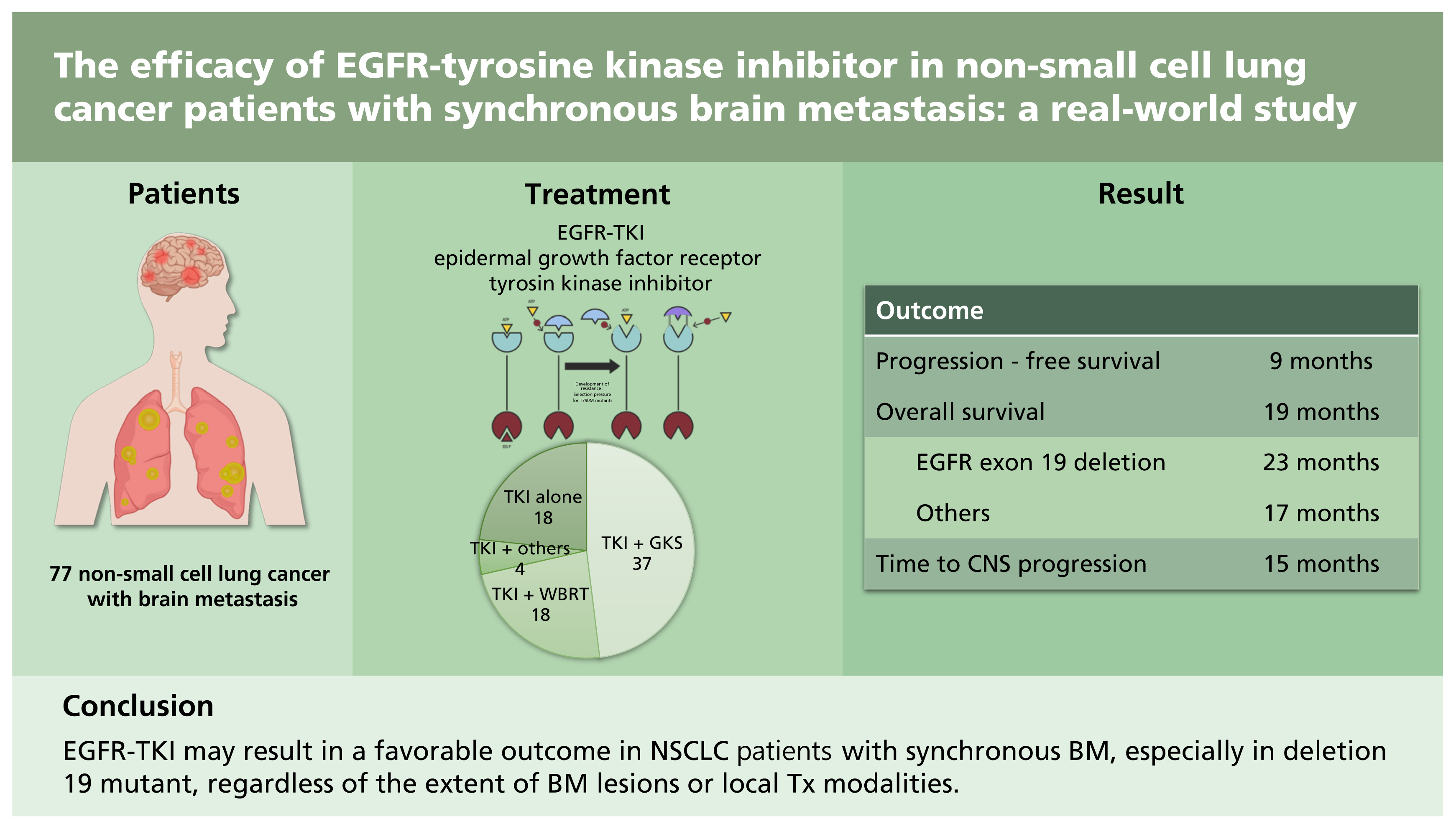
A retrospective review was conducted on 77 NSCLC patients with synchronous BM who underwent first-line EGFR-tyrosine kinase inhibitor (TKI) Tx. The outcomes of patients were analyzed according to the clinicopathological characteristics including local Tx modalities.
Results
Fifty-nine patients underwent local Tx for BM (gamma knife surgery [GKS], 37; whole brain radiotherapy [WBRT], 18; others, four) concurrently or sequentially with EGFR-TKI. Patients treated with TKI alone showed significantly lower incidence of central nervous system (CNS) symptoms. The median progression-free survival (PFS) and overall survival (OS) after the initiation of EGFR-TKI for all patients were 9 and 19 months, respectively. In 60 patients with follow-up brain imaging, the median time to CNS progression was 15 months. Patients with EGFR exon 19 deletion had a significantly longer median OS than those with other mutations including L858R (23 months vs. 17 months). Other clinical characteristics, including CNS symptoms, number of BM, and the use of local Tx were not associated with OS, as well as PFS. In terms of the local optimal Tx modality, no difference was found between GKS and WBRT in the OS and PFS.
Conclusions
This study suggests that EGFR-TKI may result in a favorable outcome in NSCLC patients with synchronous BM, especially in deletion 19 mutant, regardless of the extent of BM lesions or local Tx modalities. Patients with asymptomatic BM can be treated with EGFR-TKI and careful surveillance.
INTRODUCTION
Approximately 20% to 40% of non-small cell lung cancer (NSCLC) patients will develop brain metastasis (BM) during the course of their disease [1,2]. In several studies especially from Asia, the frequency of synchronous or metachronous BM in epidermal growth factor receptor (EGFR)-mutant NSCLC patients (39% to 70%) has been higher than that in patients with the EGFR-wild type (28% to 38%) [3–5]. Before the development of targeted therapy, the median overall survival (OS) of an unselected population of NSCLC patients with BM including EGFR-mutant was generally poor, ranging from 3 to 15 months [1,2].
Cytotoxic chemotherapeutics are largely ineffective intracranially because of their inability to cross the brain-blood-barrier. Therefore, the standard treatment for BM has traditionally been local intervention such as surgical resection, whole brain radiotherapy (WBRT), and stereotactic radiosurgery (SRS), with or without additional chemotherapy [1,2,6,7]. Since the introduction of EGFR-tyrosine kinase inhibitor (EGFR-TKI), several studies have shown that EGFR-mutant NSCLC patients with BM could achieve favorable outcomes when treated with EGFR-TKIs [7,8]. However, the optimal treatment strategy, such as upfront EGFR-TKI or the addition of local treatment, remains to be determined.
Therefore, we performed a retrospective analysis to identify the potential prognostic factors and determine the optimal incorporation of local treatment in EGFR-mutant NSCLC with synchronous BM.
METHODS
Study population
We retrospectively reviewed the clinical data of primary metastatic or recurrent EGFR-mutant NSCLC patients with synchronous BM who had started EGFR-TKI as the first-line therapy at Ajou University Hospital, between July 2011 and June 2018. All patients had BM at the time of initial diagnosis or recurrence. Patients who had started first-line EGFR-TKI at other hospitals during this period were excluded. The patients received a daily EGFR-TKI treatment of 250 mg of gefitinib or 150 mg of erlotinib or 40 mg of afatinib according to the discretion of the medical oncologists. In most patients, the treatment modalities (gamma knife surgery [GKS] or surgical resection or WBRT) for BM were selected according to the decision of the multidisciplinary team, including medical oncologists, neurosurgeons, and radiation oncologists, based on the size and number of BM, age, Eastern Cooperative Oncology Group (ECOG) performance status (PS), and other individual factors. GKS was performed using a model C Leksell Gamma Knife and Leksell GammaPlan version 10.1.1 planning system (Elekta Instruments AB, Stockholm, Sweden). The responses to EGFR-TKI were evaluated every 2 to 3 months with chest computed tomography while the patients were on therapy. Brain magnetic resonance imaging was performed at 3 months after GKS then repeated every 6 months in patients who underwent GKS, whereas it was performed according to the discretion of physicians in patients treated with WBRT or EGFR-TKI alone.
All procedures performed in the study involving human participants were carried out in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The protocol was reviewed and approved by the Institutional Review Board (IRB) of Ajou University Hospital (IRB approval no. AJIRB-MED-MDB-19-394). The IRB decided to waive the informed consent for this study because it was a retrospective study using anonymized data. Some patients (n = 19) in the present study cohort was included in a previous study [9] about the prognostic significance of EGFR mutation subtypes in metastatic NSCLC, not limited to BM. However, the follow-up of the patients was extended in the current study.
Clinical review
Data on the patients were collected, including patient characteristics (gender, age, ECOG PS, histology, disease status at diagnosis, metastasis site, number of BM, central nervous system [CNS] symptoms, type of EGFR mutation, type of EGFR-TKI, and treatment modality of BM) and survival information.
Statistical analysis
OS, progression-free survival (PFS), and time to CNS progression (TTCP) were calculated using the Kaplan–Meier method. OS was defined as the time from the starting day of EGFR-TKI to death. Data on the survivors were censored at the last follow-up. PFS was defined as the time from the starting day of EGFR-TKI to disease progression or the death of any cause. TTCP was defined as the time from the start day of EGFR-TKI to CNS progression. Data of the patients alive or who died without evidence of CNS progression were censored at the last follow-up time or the date of death, respectively. The log-rank test was used to analyze the differences between the survival curves. The comparison of the categorical variables between groups was performed by Fisher’s exact test. All statistical analyses were performed two-sided using SPSS version 23.0 for Windows (IBM Co., Armonk, NY, USA).
RESULTS
Patient characteristics
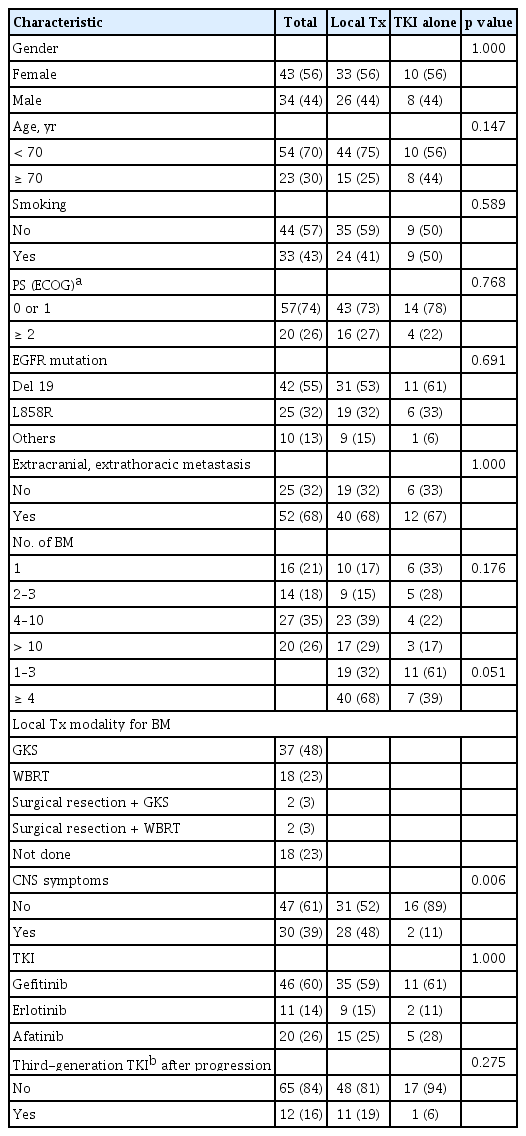
A total of 77 EGFR-mutant NSCLC with synchronous BM patients received first-line EGFR-TKI treatment at our institution. Table 1 summarizes the patients’ clinicopathological characteristics. Among the 77 patients, 43 (56%) were female, with a median age of 63 years (range, 23 to 84), 57 (74%) were in ECOG PS 0 or 1, and 44 (57%) were never smokers. The pathologic subtype was adenocarcinoma in 73 (not specified in two patients, adenosquamous carcinoma and large cell carcinoma in two patients). All patients had primary metastatic disease except one patient with recurrence, and 52 (68%) patients had extracranial and extrathoracic metastasis at the time of diagnosis. While 16 (21%) and 20 (26%) patients had single and > 10 BMs, respectively, CNS symptoms were observed in 30 patients (39%). Exon 19 deletion was the most common subtype of EGFR mutation (42 patients, 55%). The patients were treated with gefitinib (46 patients), erlotinib (11 patients), or afatinib (20 patients).
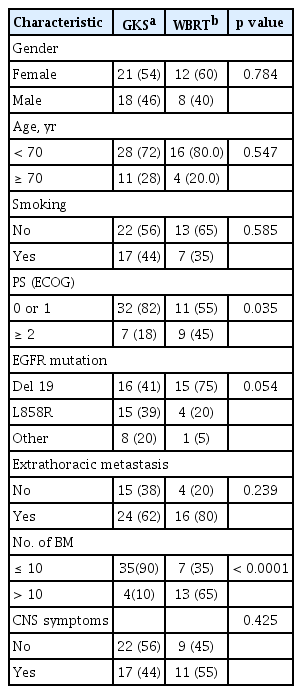
Eighteen patients (23%) were treated with TKI alone and the remaining patients received additional local treatment (GKS, 37; WBRT, 18; GKS or WBRT after surgical resection, four). The majority of patients with local treatment underwent or started the therapy before the initiation of TKI (41 patients), while 13 and five patients began the therapy after or on the same day, respectively. Patients treated with TKI alone showed a significantly lower incidence of CNS symptoms (11% vs. 48%, p = 0.006) with a tendency toward having a smaller number of BM lesions compared with those who received local treatment (Table 1). According to the local treatment modalities, the proportions of patients with poor PS and > 10 BMs were higher in patients receiving WBRT than in those who underwent GKS (Table 2).
Treatment outcome
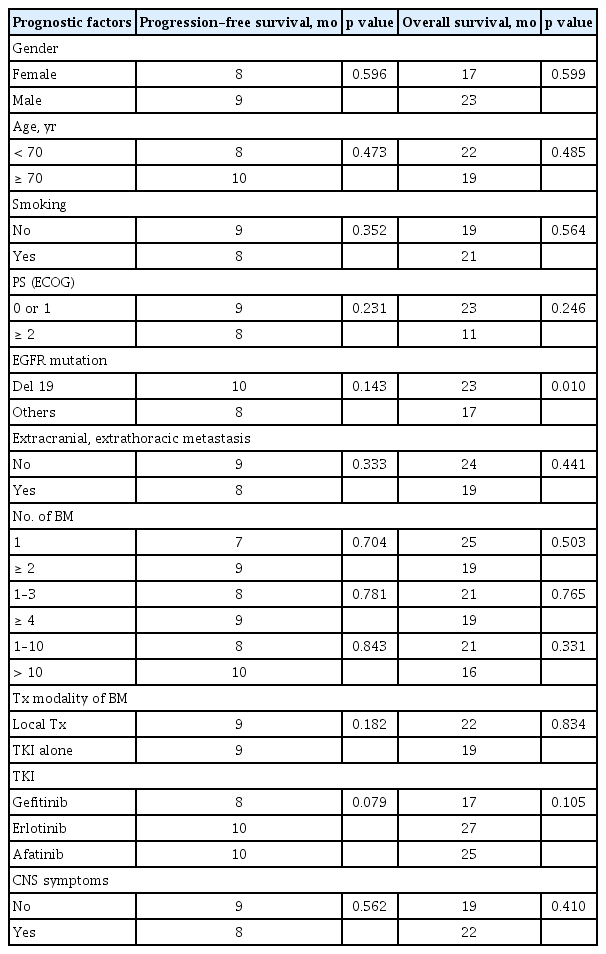
The median follow-up duration for the survivors was 32 months (range, 22 to 67). One patient was excluded for outcome analysis because of lost to follow-up after 7 days of TKI treatment. At the time of the last follow-up, 10 patients were still alive. The median PFS and OS of all patients after the initiation of TKI therapy were 9 and 19 months, respectively (Fig. 1). The median TTCP, analyzing 60 patients with available follow-up brain imaging (GKS, 37; WBRT, 14; TKI alone, nine), was 15 months (Fig. 1). The median OS of patients with exon 19 deletion was significantly longer than that of patients with other mutations (23 months vs. 17 months, p = 0.010). Other clinical characteristics, including the number of BM lesions, were not associated with the outcomes of patients (Table 3). In addition, there were no significant differences in TTCP according to the types of EGFR-TKI (gefitinib vs. erlotinib or afatinib, p = 0.170; gefitinib or erlotinib vs. afatinib, p = 0.693). The median OS, PFS, and TTCP of patients who received local treatment were not different compared with those of the patients treated with TKI alone (22 months vs. 19 months, p = 0.834; 9 months vs. 9 months, p = 0.182; 15 months vs. 19 months, p = 0.666) (Fig. 2).
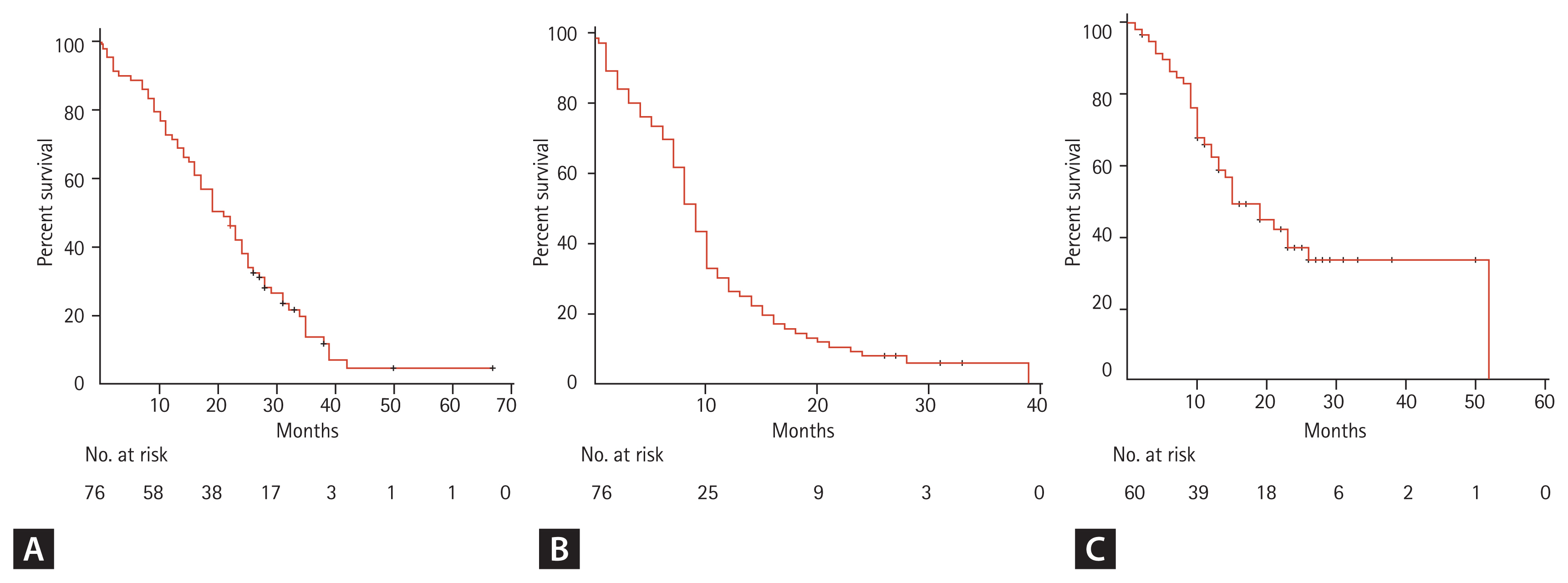
(A) Overall survival, (B) progression-free survival, and (C) time to central nervous system progression of epidermal growth factor receptor-mutant non-small cell lung cancer patients with brain metastasis.
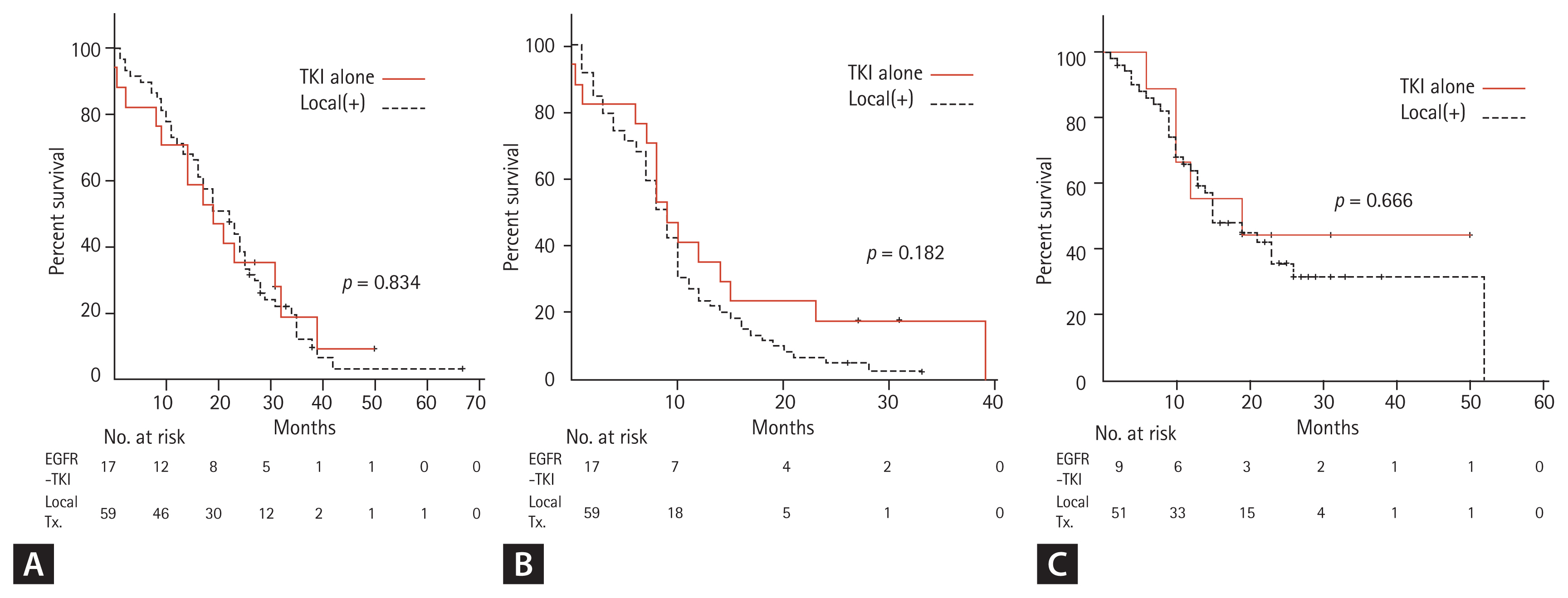
(A) Overall survival, (B) progression-free survival, and (C) time to central nervous system progression of epidermal growth factor receptor (EGFR)-mutant non-small cell lung cancer patients with brain metastasis according to treatment modality (EGFR-tyrosine kinase inhibitor [TKI] vs. local treatment [Tx]).
In the 47 patients without CNS symptoms (asymptomatic BM), 31 received local treatment, and 16 were treated with TKI alone. The median OS, PFS, and TTCP were not different between the two groups (19 months vs. 19 months, p = 0.443; 8 months vs. 10 months, p = 0.099; 15 months vs. 19 months, p = 0.692). In the 59 patients who received local treatment, no significant differences were observed between GKS and WBRT in OS (23 months vs. 16 months, p = 0.296), PFS (9 months vs. 8 months, p = 0.848), and TTCP (13 months vs. 26 months, p = 0.070). In terms of initial local treatment-associated side effects, among 51 patients with follow-up brain imaging, radiation-induced leukoencephalopathy was found in two patients receiving WBRT, with one patient having cognitive dysfunction, in addition to two patients with peritumoral edema due to progression of lesions.
DISCUSSION
The present study analyzed the outcomes of patients with EGFR-mutant NSCLC with BM who initiated first-line EGFR-TKI at our institution since 2011, when gefitinib had become reimbursable for first-line chemotherapy from the Korean National Health Insurance system. The outcomes of the current study cohort, including OS, PFS, and TTCP, were generally comparable with those of previous retrospective studies. In addition, we compared the treatment results of the present study with those of LUX-Lung 7, a randomized trial of afatinib versus gefitinib as a first-line treatment for EGFR-mutant NSCLC, with a preponderance of East Asian patients [10,11]. The median OS of the current study cohort was somewhat shorter than that of the LUX-Lung 7 trial (27.9/24.5 months) [11]. However, the LUX-Lung 7 trial included only ECOG PS 0 or 1 patients with a small proportion of patients with BM (16%), whereas the present study consisted of a significant number of patients with ECOG PS 2 or 3 (26%) [10]. Furthermore, the median OS of patients with ECOG 0 or 1 (23 months) in the present study was almost similar to that of the LUX-Lung 7 trial [11]. Moreover, the median PFS of the present study cohort (9 months) was comparable to that of patients with BM (7.2/7.4 months) in the LUX-Lung 7 trial [10]. These findings suggest that the current treatment outcomes of real-world practice are at least not inferior to those of clinical trial that consisted of selected patients with a relatively good health status, despite the limitation of an indirect comparison of two studies.
The current study demonstrated that exon 19 deletion was the only prognostic factor associated with favorable OS among various clinical characteristics. In patients with BM as well as unselected population of EGFR-mutant metastatic NSCLC including the cohort of our previous study, several studies showed better OS in patients with exon 19 deletion than in those with other mutations such as L858R [1,9,12–14].
The most controversial issue in the management of EGFR-mutant NSCLC with BM is the incorporation of local treatment. Although many retrospective studies showed conflicting results [5–8,15–20], a few meta-analyses revealed better OS by addition of local treatment [2,21,22]. Furthermore, a large retrospective study from the United States demonstrated improved OS using upfront local treatment compared with EGFR-TKI alone [1]. The benefit of additional local treatment in NSCLC patients with BM treated with EGFR-TKI has the following biologic basis. First, many studies have shown that EGFR-mutant NSCLC is highly radiosensitive in both clinical and preclinical settings [1,23]. Second, radiation can disrupt blood-brain barrier, leading to increased penetration and cerebrospinal fluid concentration of EGFR-TKI [5,20–22]. Third, EGFR-TKI may radiosensitize NSCLC cells [17,22]. Finally, due to the discordance of EGFR mutations between the primary tumor and BM in some patients, EGFR-TKI can be ineffective to EGFR-wild type BM lesions [2,5].
In terms of local modalities, SRS, such as GKS, combined with EGFR-TKI, seems to be the best approach because of its convenience and less toxicity, such as in neurocognitive dysfunction, and because several studies have indicated that it showed better OS than WBRT [1,2,21,22,24,25]. However, in the present study, no difference was found in OS, PFS, and TTCP between GKS and WBRT despite the higher proportion of patients with poor PS and multiple BM lesions in the WBRT group. These results suggest that WBRT can be considered a useful alternative for patients unsuitable for SRS.
In contrast to previous large studies showing the advantage of additional local treatment [1,2,17,20–22], no significant difference was found in the OS, PFS, and TTCP between patients who underwent local treatment and those who were treated with EGFR-TKI alone in the present study. The significantly higher proportion of asymptomatic BM patients in the EGFR-TKI alone group and the absence of a significant difference in the outcomes between the local treatment and EGFR-TKI alone groups in patients without CNS symptoms may explain such results. Several retrospective studies have investigated the benefit of adding local therapy to EGFR-TKI in EGFR-mutant NSCLC patients with asymptomatic synchronous BM, but they showed conflicting results in terms of OS [8,16,18,19]. However, direct comparison with the current study seems to be difficult because some of these ones analyzed patient cohorts treated with WBRT alone [18] or including second or further line of EGFR-TKI therapy [8,19]. Upfront EGFR-TKI may be a useful option for certain subgroups of EGFR-mutant NSCLC patients with BM such as those having no CNS symptom or a smaller number of BM lesions, given its antitumor activity in BM in addition to extracranial lesions [5,7,20]. Furthermore, the initiation of systemic treatment is not delayed with the avoidance of potential toxicity of local treatment [1]. However, a prospective randomized trial is essential to define the optimal treatment strategy for EGFR-mutant NSCLC patients with BM, although it seems to be difficult to conduct. Given that recently published FLAURA study demonstrated that osimertinib had better CNS efficacy than first-generation EGFR-TKIs in patients with asymptomatic or stable CNS metastases [26], upfront third-generation TKI may be consider as the standard treatment option for patients with asymptomatic or stable BM. In patients who receive upfront EGFR-TKI, close surveillance with brain imaging seems to be necessary to conduct a timely local treatment in case of CNS progression.
This study analyzed all EGFR-mutant NSCLC patients with synchronous BM who had started EGFR-TKI as the first-line therapy in a single institution, during the defined period with a mature follow-up. Whereas many previous studies investigated WBRT alone cohorts or rather heterogenous population including second or more line of EGFR-TKI and metachronous BM [7,8,17–19], the current study analyzed only synchronous BM patients treated with first-line EGFR-TKI with more frequent usage of GKS than WBRT. Therefore, the results of the present study can reflect the treatment outcomes of real-world clinical practice in Korea.
However, this study has several limitations. First, this work is a retrospective analysis from a single institution with a relatively small sample size including patients treated with three types of EGFR-TKI. Second, the patients treated with local treatment only or best supportive care were not included in the analysis. Third, given the retrospective nature of the analysis, we did not fully analyze the potential toxicities such as neurocognitive dysfunction associated with local treatment and their impact on quality of life. Fourth, because follow-up brain imaging was performed according to the discretion of the physician in many cases, not all patients were evaluated for TTCP. The main reason for not performing follow-up brain imaging seems to be early extracranial progression or death, considering poor outcome of these patients (median PFS and OS: 5 and 7 months, respectively). Finally, a relatively small number of patients treated with TKI alone, mostly asymptomatic, may be the potential bias in the interpretation of results.
In conclusion, EGFR-TKI may result in favorable outcome in NSCLC patients with synchronous BM, especially in deletion 19 mutant, regardless of the extent of brain metastatic lesions or local treatment modalities. In addition, patients with asymptomatic BM can be treated with EGFR-TKI alone and careful surveillance.
KEY MESSAGE
1. Even in patients with brain metastasis, exon 19 deletion is a favorable prognostic factor.
2. Epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor (TKI) may result in a favorable outcome in non-small cell lung cancer patients with synchronous brain metastasis.
3. Asymptomatic brain metastasis can be treated with EGFR-TKI and careful surveillance.
Notes
This work was supported in part by Samyang Biopharmaceuticals Corporation, Korea. The funder did not have any role in the design and conduct of study, the analysis and interpretation of the data, decision to publish, and preparation of the manuscript.