Blood pressure control in patients with chronic kidney disease
Article information
Abstract
Uncontrolled blood pressure (BP) in patients with chronic kidney disease (CKD) can lead to serious adverse outcomes. To prevent the occurrence of cardiovascular events (CVEs), and end-stage kidney disease, achieving an optimal BP level is important. Recently, there has been a paradigm shift in the management of BP largely as a result of the Systolic Blood Pressure Intervention Trial (SPRINT), which showed a reduction in CVEs by lowering systolic BP to 120 mmHg. A lower systolic blood pressure (SBP) target has been accepted by the Kidney Disease: Improving Global Outcomes (KDIGO) 2021 guidelines. However, whether intensive control of SBP targeting < 120 mmHg is also effective in patients with CKD is controversial. Notably, this lower target SBP is associated with a higher risk of adverse kidney outcomes. Unfortunately, there have been no randomized controlled trials on this issue involving only patients with CKD, particularly those with advanced CKD. In this review, we discuss the optimal control of BP in patients with CKD in terms of reduction in death and CVEs as well as attenuation of CKD progression based on the evidence-based literature.
INTRODUCTION
Chronic kidney disease (CKD) and hypertension are closely connected and affect each other. Hypertensive kidney disease is the second most common cause of kidney failure with replacement therapy (KFRT), and deterioration of kidney function is accelerated by excessive high blood pressure (BP) [1-4]. Uncontrolled hypertension can cause adverse cardiovascular and cerebrovascular outcomes such as acute coronary syndrome, hemorrhagic and ischemic stroke, heart failure, and even death [5-10]. Therefore, in clinical practice, physicians typically prioritize BP control to preserve kidney function and reduce the rate of cardiovascular events (CVEs) and mortality in patients with CKD.
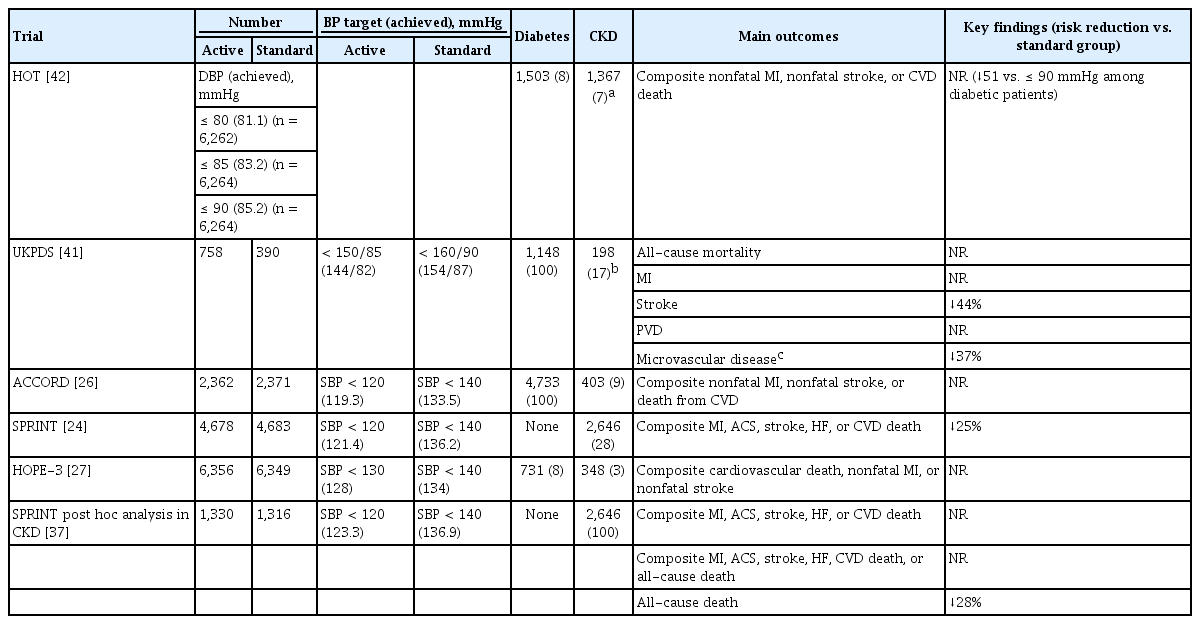
Therefore, the question for consideration is, “What is the optimal target BP that will achieve these goals in patients with CKD?” Unfortunately, this issue has not yet been resolved. In addition to reducing the rate of adverse CVEs and mortality in patients with and without CKD, other important goals of BP control are to prevent the development of CKD for non-CKD patients and to attenuate the deterioration of kidney function in patients with CKD. Epidemiologic studies have shown that, in patients without CKD, even a pre-hypertension level of systolic blood pressure (SBP; 130 to 140 mmHg) is associated with a higher risk of CKD development compared with an SBP of < 120 mmHg [11-14]. In addition, a meta-analysis of observational data showed a higher risk of CVEs in individuals with an SBP of 120 to 129 mmHg compared to those with an SBP of < 120 mmHg [15]. Notably, guidelines from various countries do not agree on BP control in patients with CKD [16-22]. Over the last two decades, < 140/90 mmHg has typically been the target BP in patients without albuminuria and < 130/80 mmHg in those with albuminuria [17,23]. This conventional concept has been challenged by the results of the Systolic Blood Pressure Intervention Trial (SPRINT) study, which demonstrated the benefit of intensive control of SBP < 120 mmHg compared with the conventional target of < 140 mmHg [24]. The SPRINT study results are included in the Kidney Disease: Improving Global Outcomes (KDIGO) 2021 guidelines, which suggest a target SBP of < 120 mmHg in patients with CKD [25]. However, not all studies favor this lower target, given the negative results of intensive BP control [26-28]. In fact, some guidelines still recommend the conventional BP target for patients with CKD [29-31]. BP targets in patients with CKD are listed in Table 1 [18,19,22,25,32-35].
In this article, we discuss the following three goals of optimal BP control in patients with CKD: (1) to prevent CVEs and all-cause death, (2) to prevent the development of incident CKD, and (3) to delay the progression of CKD. Finally, we briefly describe conventional and potential drug therapies for improving outcomes in patients with CKD.
BP MEASUREMENT
The KDIGO 2021 BP guidelines recommend use of standardized rather than routine office BP measurement [25], as suggested by the 2017 American College of Cardiology/American Heart Association (ACC/AHA) guidelines [32]. Standardized office BP measurement refers to BP measurement following the recommended preparation procedure. A summary of standardized office BP measurement is provided in Table 2 [32]. In contrast, routine office BP measurement is performed without considering the recommended BP measurement procedure. Notably, BP readings using office BP measurement are typically higher than those using standardized BP measurement [36]. BP measurement procedures vary among clinical trials, a critical point that should be considered when interpreting study results. However, for the sake of convenience, routine office BP measurement is commonly used in the real world. Given the possible risk of overtreatment and hypotension events associated with office BP measurement, use of standardized BP measurement should be encouraged in clinical practice.
BP CONTROL FOR PREVENTION OF CARDIOVASCULAR DISEASE AND DEATH IN CKD
Given the lack of randomized controlled trials (RCTs) in patients with only CKD, this issue has been evaluated by analyses of secondary outcomes. As mentioned above, the SPRINT study demonstrated that intensive control of SBP to < 120 mmHg resulted in better cardiovascular outcomes compared to control to < 140 mmHg [24]. This finding has been adopted by the ACC/AHA 2017 guidelines [16] and the KDIGO 2021 guidelines [25]. Notably, the SPRINT study excluded patients with diabetes and a history of stroke. Most participants with CKD had CKD G3a; their mean estimated glomerular filtration rate (eGFR) was 48 mL/min/1.73 m2. Therefore, it is unclear whether intensive control using a lower BP target is beneficial in other risk groups, such as patients with diabetes or CKD G3b, G4, or G5. In the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial involving patients with type 2 diabetes, intensive control of SBP < 120 mmHg did not reduce the rate of a composite outcome of fatal and nonfatal major CVEs or death as compared with a standard target (< 140 mmHg), although intensive control decreased the rate of nonfatal stroke events [26]. Nevertheless, 2,646 participants with an eGFR of < 60 mL/min/1.73 m2 in the SPRINT study provided adequate statistical power to examine the effect of intensive BP control on cardiovascular outcomes in patients with early CKD. In this separate analysis of participants with CKD, the intensive SBP intervention group had a 19% lower risk of composite cardiovascular outcomes and a 28% lower risk of all-cause death compared to the standard BP control group [37]. In addition, in a meta-analysis of 123 trials including 613,815 participants with BP lowering intervention, each 10 mmHg reduction in SBP significantly decreased the risk of major CVEs and all-cause mortality [38]. Notably, a greater reduction in SBP resulted in a larger decrease in the relative risk of CVEs. That study also showed a significant risk reduction with a decrease in SBP in patients with CKD. However, a pooled analysis of four multicenter RCTs including 4,983 CKD patients with control of SBP to < 130 mmHg did not improve all-cause mortality and cardiovascular outcomes compared to standard control of SBP to < 140 mmHg [39]. In that study, the average BP achieved was 125.0 mmHg in the intensive group and 136.9 mmHg in the standard group. After excluding patients with an eGFR of ≥ 60 mL/min/1.73 m2 and intensive glucose control, the all-cause mortality rate was reduced in the intensive BP control group. Finally, in another meta-analysis of patients with CKD G3–G5, more intensive control (SBP 132 mmHg) resulted in a 14.0% lower risk of all-cause mortality compared with less intensive control (SBP 140 mmHg) [40]. Therefore, the evidence supports intensive BP control to reduce adverse CVE and all-cause mortality rates, even in patients with CKD. A summary of these RCTs is presented in Table 3 [24,26,27,37,41,42]. In contrast to RCTs, analyses of observational cohort studies have shown a U-shaped association between SBP and the risk of death in patients with CKD, suggesting a potential hazard for excessively low BP [43-46]. A low BP itself may reflect an unhealthy condition and underlying disease burden.
BP CONTROL FOR THE PREVENTION OF CKD
No RCT has examined the effect of intensive BP control on the development of incident CKD. Studies on this issue are mostly post hoc analyses of RCTs comparing the effect of intensive versus standard BP control in patients with high cardiovascular risk. Among these, the ACCORD trial showed that intensive control of SBP < 120 mmHg increased the risk of incident CKD [26]. In line with this study, a similar SBP intervention in the SPRINT study resulted in a higher frequency of adverse kidney outcomes among patients without diabetes [24]. Therefore, intensive BP control is not beneficial for preserving kidney function. However, intensive BP control is effective in reducing albuminuria. Despite the increased risk of CKD, the ACCORD trial showed a significant reduction in macroalbuminuria by intensive BP control. In a similarly designed RCT involving patients with type 2 diabetes, active BP control significantly reduced the risk of microalbuminuria and macroalbuminuria [47,48]. A reduction in albuminuria by intensive BP control was also found in a meta-analysis of 19 RCTs including both diabetic and non-diabetic patients [49]. For the prevention of CKD, these studies indicate that intensive BP control carries both a risk and a benefit: a decline in eGFR and reduction in albuminuria. To mitigate concerns on the increase in the risk of adverse kidney outcomes by intensive BP control, the SPRINT and ACCORD investigators showed that various kidney injury markers were not elevated in the intensive BP control group, suggesting that the increased serum creatinine level was associated with hemodynamic alterations [50,51]. Despite the debate on its validity as a surrogate marker of renal function, albuminuria is widely accepted as an appropriate target or surrogate marker for kidney disease progression [52]. The United States Food and Drug Administration recently agreed to use early change in albuminuria as a surrogate marker for kidney disease progression in phase 3 trials dealing with diseases involving moderate to severe albuminuria, and intervention trials in which decreasing albuminuria is presumed to be the primary mechanism of action [53]. The key findings of major RCTs are shown in Table 4.
In contrast to previous RCTs involving patients with an increased risk of CVD and a baseline SBP of ≥ 130 mmHg, observational studies provide comprehensive information on individuals at low risk of CKD and few comorbidities, even those without pre-existing hypertension. Interestingly, there was a graded relation ship between SBP and the risk of incident CKD, and the risk was lower for an SBP of < 120 mmHg [11-13,54,55]. We observed similar findings in two large representative Korean adult cohorts using meticulous analytical approaches [56,57]. These findings raised the question of whether reducing SBP to < 120 mmHg in individuals with hypertension and at high risk of CVD is equivalent to an intrinsically normal BP in healthy adults. In summary, intensive BP control for the prevention of incident CKD has not yet been justified. If kidney injury associated with intensive BP control is minimal and reversible, a lower BP target can be implemented in clinical practice with a permissive decline in eGFR given the proven benefit on prevention of adverse CVEs.
BP CONTROL FOR DELAYING THE PROGRESSION OF CKD
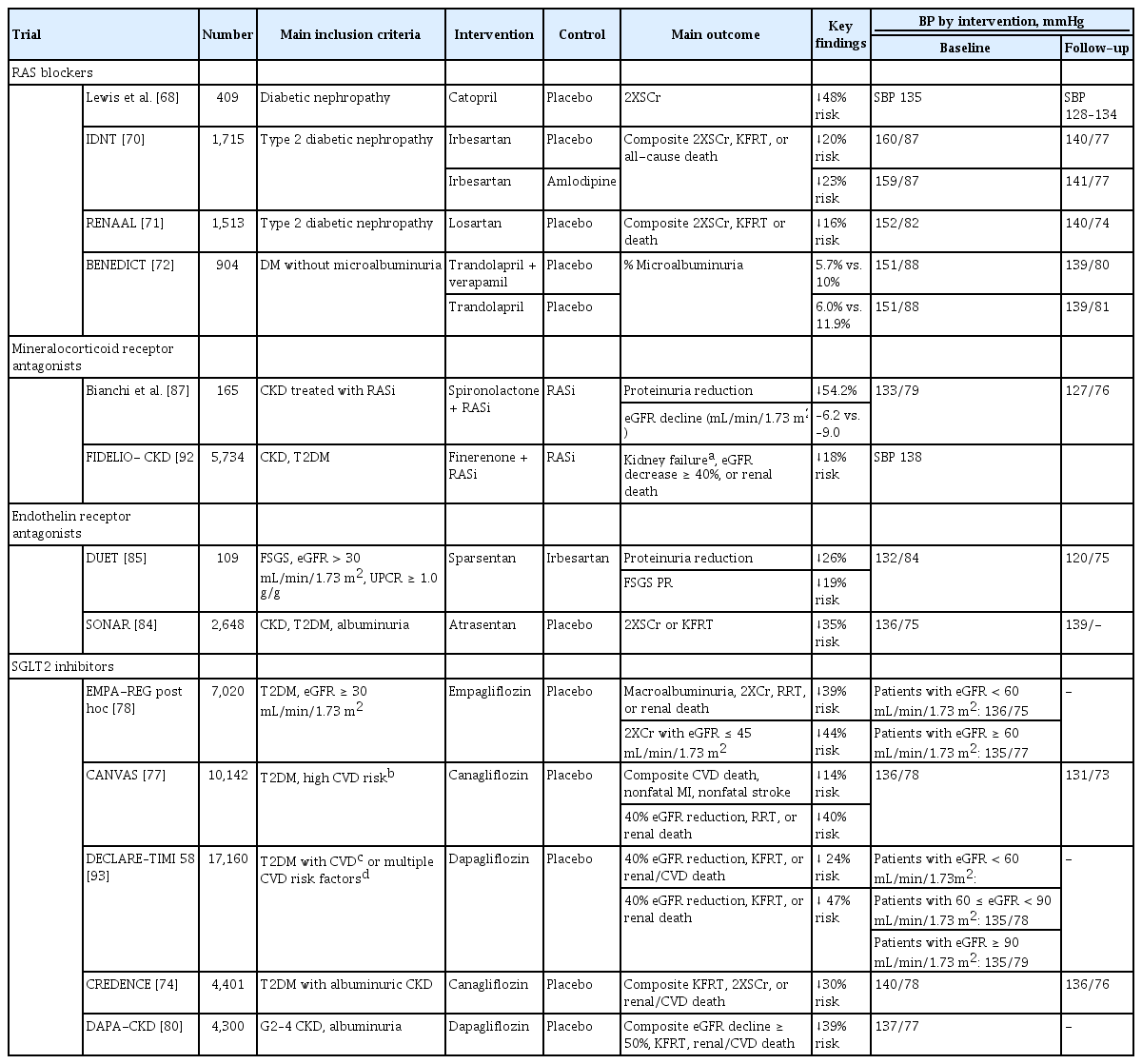
CKD progression is generally faster in patients with than in those without diabetes. In non-diabetic patients with CKD, three clinical trials have evaluated the effect of intensive BP control on CKD progression: the Modification of Diet in Renal Disease (MDRD) study; the AASK trial; and the Blood Pressure Control for Renoprotection in Patients with Non-diabetic Chronic Renal Disease (REIN-2) trial [28,58,59]. The African-American Study of Kidney Disease and Hypertension (AASK) and REIN-2 trials excluded patients with diabetes. Only 5% of participants in the MDRD study were patients with diabetes. The target BPs in the intensive control arm were < 125/75 mmHg (mean arterial pressure < 92 mmHg) for the MDRD study and < 130/80 mmHg for the AASK and the REIN-2 trials. The MDRD study, published in 1994, showed that the beneficial effect of a lower BP target was particularly evident in patients with daily urinary protein excretion > 1.0 g [59]. Long-term follow-up of the MDRD study with observation extended to 10 years also showed that CKD outcomes and the all-cause mortality rate were decreased by intensive BP control [60]. However, the AASK and REIN-2 trials failed to delay the progression of CKD or the development of KFRT in patients with intensive BP control. Notably, in the AASK trial, a subgroup of patients with CKD and a protein-to-creatinine ratio of ≥ 0.22 g/g in the intensive BP control group had a significant reduction in the risk of KFRT or death. These findings suggest that intensive BP control is more effective in non-diabetic patients with CKD and significant proteinuria. A systemic review and meta-analysis supports the ability of intensive control of BP < 130/80 mmHg to attenuate CKD progression in non-diabetic patients with CKD and significant proteinuria [61,62].
The clinical benefits of intensive BP control are unclear in patients with type 2 diabetes. The BP goals in most early studies were > 140 mmHg, which was considered suboptimal [41,42,63,64]. The Appropriate Blood Control in Diabetes (ABCD) study evaluated the effect of a lower BP target of < 130/80 mmHg on the preservation of kidney function in patients with type 2 diabetes compared with a standard control of < 140/90 mmHg but failed to demonstrate a benefit of intensive BP control [65]. However, two post hoc analyses of the Reduction of Endpoints in Non-Insulin-Dependent Diabetes Mellitus with the Angiotensin II Antagonist Losartan (RENAAL) and the Irbesartan Diabetic Nephropathy Trial (IDNT) showed that a lower BP level was associated with improved kidney outcomes [66,67]. In contrast to the less-intensive BP targets in the studies above, the intervention in the ACCORD trial lowered SBP to < 120 mmHg [26]. However, as discussed above, a lower target BP increased the frequency of adverse kidney events such as an elevated serum creatinine level or a decline in eGFR of < 30 mL/min/1.73 m2. It should be noted that the ACCORD study excluded patients with a serum creatinine level of > 1.5 mg/dL; therefore, the participants were unlikely to be representative of patients with CKD and diabetes. We have summarized the results of major RCTs on this topic in Table 4 [24,26,28,47,58,59,65-67].
DRUG TREATMENT
Besides achieving the optimal BP, choosing medications with renoprotective effects is important. A detailed description of this issue is beyond the scope of this review. Here, we briefly describe conventional drugs and introduce several with potential for prevention of CKD progression.
Renin-angiotensin system blockers (RASBs), such as angiotensin-converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARB), are a cornerstone therapy in patients with CKD. In a groundbreaking study by Lewis et al. [68], captopril was first used to protect against a decline in kidney function in patients with insulin-dependent type 1 diabetes and CKD studies with RASBs have consistently demonstrated the renoprotective effects of these drugs, including a reduction in proteinuria and attenuation of eGFR decline. These beneficial effects were evident in patients with and without diabetes. In the AASK trial, patients randomly assigned to ramipril showed a slower decline in eGFR compared to those on other treatments [69]. In patients with type 2 diabetes and CKD, the RENAAL and IDNT studies confirmed the superior protective effects of ARBs against the progression of CKD [70,71]. RASBs have been tested in patients with early CKD, even those without microalbuminuria. In the Bergamo Nephrologic Diabetes Complications Trial (BENEDICT), ACEi prevented the onset of microalbuminuria in patients with type 2 diabetes and normal urinary albumin excretion [72]. All guidelines recommend the use of RASBs as first-line therapy based on high-quality evidence [18,19,22,25,32-34].
RASBs cannot stop the progression of CKD and no other drugs are used widely in clinical practice. Recently, many studies with new anti-diabetic drugs, such as sodium-glucose co-transporter 2 inhibitors (SGLT2i) and glucagon-like peptide-1 receptor agonists (GLP1RA), have consecutively demonstrated outstanding renoprotective effects [73,74]. The American Diabetes Association (ADA), the European Association for the Study of Diabetes (EASD), and the KDIGO accepted the results of these studies and recommend SGLT2i and GLP1RA as firstline therapies for patients with diabetic kidney disease [75,76]. Interestingly, these drugs have also been reported to reduce adverse CVEs and death [77-79]. Moreover, in the Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) trial, dapagliflozin reduced the risk of the composite outcome of kidney failure or death from renal or cardiovascular causes even in non-diabetic patients with CKD [80]. In addition, these drugs can reduce BP by 5 mmHg [79,81,82].
Atrasentan is a highly selective endothelin receptor A receptor (ETAR) antagonist, the short-term use of which reportedly reduces albuminuria in patients with diabetic nephropathy [83]. The Study of Diabetic Nephropathy with Atrasentan (SONAR) tested the long-term effect of atrasentan in 2,648 patients with type 2 diabetes and overt albuminuria [84]. Atrasentan resulted in a significant reduction in the composite adverse kidney outcome of doubling serum creatinine or KFRT. A recent phase 2 study of sparsentan, a dual ETAR antagonist and ARB, also showed a significant reduction in proteinuria in patients with primary focal segmental glomerulosclerosis [85]. ETAR antagonists are reported to decrease BP [85,86]. The effects of ETAR antagonists are under investigation in other primary glomerular diseases (NCT03762850, NCT03493685, NCT04573478).
Finally, mineralocorticoid receptor antagonists (MRAs) have renoprotective and cardioprotective effects [87-89]. It also reduces BP in individuals with resistant hypertension [90,91]. Spironolactone, a first-generation nonselective MRA, was initially reported to provide renoprotective effects by reducing proteinuria and preserving eGFR in non-diabetic patients with CKD [87]. Recently, finerenone, a new-generation selective MRA, has emerged as a potential therapy. In the Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease (FIDELIO-DKD) study, finerenone reduced the risk of CKD progression and the development of CVEs in patients with CKD and type 2 diabetes [92]. These drugs are promising and expected to improve kidney survival and major clinical outcomes in combination with RASBs. Table 5 lists clinical trials of drug treatments [68,70-72,74,77,78,80,84,85,87,92,93].
CONCLUSIONS
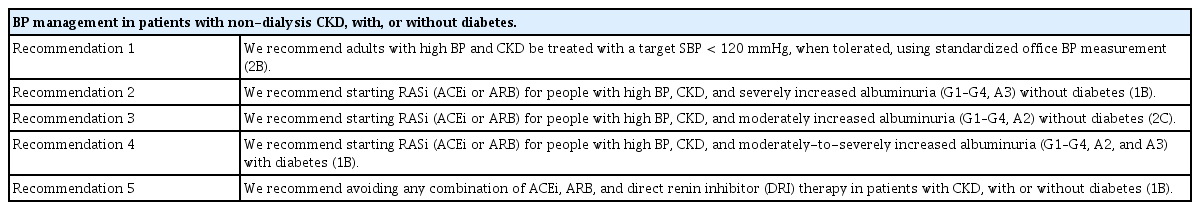
In patients with CKD, the scope of ‘optimal BP control’ should encompass improved cardiovascular outcomes, reduced mortality, and delayed CKD progression. However, despite much research, the optimal BP reduction to achieve these goals has not been determined. Table 6 presents a summary of BP control based on the KDIGO 2021 guidelines. These recommendations and suggestions are helpful in clinical practice, and the guidelines support intensive BP control targeting an SBP of < 120 mmHg because the cardiovascular benefits of SBP intervention outweigh the risk of kidney injury associated with the lower BP target. However, many uncertainties remain to be resolved in future trials. We anticipate that a greater number of well-designed RCTs will assess the effects of intensive BP control by various interventions in diverse groups of patients with CKD with and without diabetes, a high cardiovascular risk, or proteinuria, and with early versus late CKD.
Notes
No potential conflict of interest relevant to this article was reported.