Risk stratification of patients with gastric lesions indefinite for dysplasia
Article information
Abstract
Background/Aims
There are no definite guidelines for the management of gastric lesions diagnosed as indefinite for dysplasia (IND) by endoscopic forceps biopsy (EFB). Therefore, this study aimed to evaluate the clinical outcomes of gastric IND and predictive factors for gastric neoplasm.
Methods
This study included 457 patients with a first diagnosis of gastric IND by EFB between January 2005 and December 2013. Patient characteristics and endoscopic and pathological data were reviewed and compared.
Results
Of the 457 gastric IND patients, 128 (28%) were diagnosed with invasive carcinoma, 21 (4.6%) with high-grade dysplasia, 31 (6.8%) with low-grade dysplasia, and 277 (60.6%) as negative for dysplasia. Of lesions observed, 180 (39.4%) showed upgraded histology. Multivariate analysis revealed that surface erythema (odds ratio [OR], 2.804; 95% confidence interval [CI], 1.741 to 4.516), spontaneous bleeding (OR, 2.618; 95% CI, 1.298 to 5.279), lesion size ≥ 1 cm (OR, 5.762; 95% CI, 3.459 to 9.597), and depressed morphology (OR, 2.183; 95% CI, 1.155 to 4.124) were significant risk factors for high-grade dysplasia or adenocarcinoma. The ORs associated with 2 and ≥ 3 risk factors were 7.131 and 34.86, respectively.
Conclusions
Precautions should be taken in the management of gastric IND patients, especially when risk factors, including surface erythema, spontaneous bleeding, lesion size ≥ 1 cm, and depressed morphology are present. Considering the combined effect of the presence of multiple risk factors on the incidence of high-grade dysplasia or adenocarcinoma, endoscopic resection should be recommended if a gastric IND patient has at two or more of these factors.
INTRODUCTION
Gastric adenocarcinoma progresses through an inflammation-metaplasia-dysplasia-carcinoma sequence, which is described as the Correa cascade of gastric carcinogenesis [1]. Management of precancerous lesions is based on the understanding that these lesions develop into gastric cancer through a stepwise progression of histopathological stages, and gastric epithelial dysplasia is thought to be a direct precursor of gastric adenocarcinoma [2]. According to the revised Vienna classification [3], gastric epithelial dysplasia is classified into five groups: negative for neoplasia/dysplasia (category 1); indefinite for neoplasia/dysplasia (category 2); mucosal low-grade neoplasia/dysplasia (LGD) (category 3); mucosal high-grade neoplasia/dysplasia (HGD) (category 4); and submucosal invasion by carcinoma (category 5).
Endoscopic forceps biopsy (EFB) is crucial for grading preneoplastic gastric lesions and determining an appropriate treatment strategy. However, considerable discrepancies have been found between diagnoses based on EFB and diagnoses based on subsequent endoscopic resection, because EFB specimens do not always reflect the predominant histopathology of the entire lesion [4-6]. This limitation of EFB and inflammatory reactions of lesions may lead to a diagnosis of “indefinite for dysplasia” (IND). Gastric IND on EFB represents a heterogeneous histological group and is used by pathologists when the observed architectural and nuclear abnormalities are less marked than those seen in true dysplasia but in which such changes are at the severe end of the regenerative spectrum [7].
Although gastric IND is commonly diagnosed in general practice by EFB, no definite guidelines for the management of this condition are available. Endoscopic follow-up with repeat biopsy sampling is generally recommended [3]. However, clinicians may have difficulty determining a management strategy for gastric IND patients because of concerns, such as patient compliance, cost-effectiveness, and delayed diagnosis of malignancy. Therefore, it is important to elucidate the clinical outcomes of gastric IND and assess the efficacy of active management strategies such as endoscopic resection. Sparse data currently exist that characterize clinical outcomes for patients with gastric IND diagnosed by EFB [8,9]. Moreover, it is unclear which factors are associated with gastric epithelial neoplasia, especially in the case of HGD or adenocarcinoma. Therefore, this study aimed to evaluate clinical outcomes and endoscopic and histologic risk factors for gastric IND in terms of progression to HGD or gastric adenocarcinoma.
METHODS
Patients
This study included 579 patients with gastric IND treated between January 2006 and December 2013 at Soonchunhyang University, Cheonan Hospital in Korea. All lesions had been pathologically proven or suspected as gastric IND by EFB performed at our hospital. Gastric IND was defined as the presence of cytological changes similar to those observed in dysplasia, but with surface maturation or presence of inflammation [10]. Using this definition, pathologic findings on EFB including “indefinite dysplasia,” “atypia,” and “atypical gland” were searched. Of the original population, 122 patients were excluded owing to loss of follow-up (n = 72) or endoscopic follow-up without repeated pathologic confirmation (n = 50). Thus, 457 patients with gastric IND diagnosed by EFB were analyzed.
To identify predictive factors of gastric neoplastic lesions, especially HGD (category 4) and invasive carcinoma (category 5), we reviewed clinical and endoscopic characteristics of lesions from the patient medical records and endoscopic images. The enrolled patients were divided into Vienna category 1 to 3 and category 4 to 5 groups, using histological results from follow-up endoscopic biopsy, endoscopic resection, and surgical resection. Written informed consent was obtained from each participant or a responsible family member after the possible adverse events of the diagnostic procedures were fully explained. All procedures involving human participants were performed in accordance with the ethical standards of the institutional and/or national research committees and with the 1964 Declaration of Helsinki and its later amendments, or comparable ethical standards. Due to the retrospective nature of this study, patients’ consent to participate was waived in accordance with the Institutional Review Board.
Endoscopic evaluation and forceps biopsy
Diagnostic endoscopy was performed in patients with upper gastrointestinal symptoms or for screening. Standard endoscopes with a single channel (GIF-Q260, GIF-H260, and GIF-J260, Olympus Optical, Tokyo, Japan) were used for the examinations. If gastric epithelial neoplasia was suspected, chromoendoscopy was routinely performed to identify the tumor shape and margin after spraying the lesion with 0.1% to 0.5% indigo carmine. If dysplasia or carcinoma was suspected, 2 to 6 biopsy specimens were typically obtained using standard biopsy forceps (FB-21K-1, Olympus).
Endoscopic images and reports were reviewed by a single expert endoscopist (I.K.C.). Before EFB, the size of the lesion was estimated macroscopically using standard biopsy forceps with a 6-mm opening diameter (FB-21K-1, Olympus). Using the Paris classification, tumor morphology was classified as elevated, flat, or depressed [11]. When the lesion was depressed lower than the surrounding mucosa, regardless of its shape, it was defined as a depressed-type lesion. Spontaneous bleeding was defined as bleeding before forceps biopsy or bleeding from a weak touch, such as that of a water spray. The color of the lesion was evaluated by comparing it to that of the adjacent mucosa. Whitish discoloration was defined as whitish mucosal discoloration, and surface erythema was defined as a case with ≥ 50% of the lesion colored [12]. From the endoscopic findings, tumor location (long axis) was classified by dividing the stomach into three equal sections: upper (cardia, fundus, upper body), middle (mid-body, lower body, angle), and lower (antrum, prepylorus). Circumferential locations of the lesion were described as anterior wall, posterior wall (PW), lesser curvature, and greater curvature (GC).
Histopathological evaluation
Histological diagnoses of all biopsy and resection specimens were classified according to the Vienna classification of tumors of the digestive system [3,13]. In all cases, the diagnoses of gastric IND on the first EFB were confirmed by two pathologists in our hospital. In cases of discrepancy between the two pathologists, final diagnoses were made after discussion. Neoplastic lesions were histologically classified into four categories: LGD, HGD, differentiated adenocarcinoma, and undifferentiated adenocarcinoma. The presence of atrophy or intestinal metaplasia in the surrounding gastric mucosa was evaluated histologically. Helicobacter pylori infection was considered positive if the results of endoscopic gastric mucosal biopsy, rapid urease test, or urea breath test were positive.
Statistical analysis
The significance of differences was determined using chi-square or logistic regression analysis, as appropriate. Additional multiple logistic regression analysis was performed to identify independent factors associated with the development of gastric neoplasms. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to estimate the relative risk of histological discrepancy. A p value of < 0.05 was considered statistically significant. Continuous data without normal distribution are reported as medians. All data were analyzed using the SPSS version 17.0 for Windows (SPSS Inc., Chicago, IL, USA).
RESULTS
Clinical outcomes of gastric IND
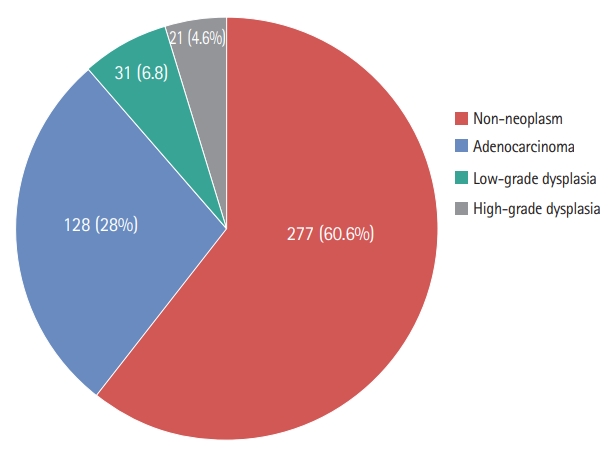
In total, 579 patients diagnosed with gastric IND by EFB were enrolled in this study. As shown in Fig. 1, 122 patients who did not undergo follow-up endoscopy or pathologic confirmation were excluded. The 457 remaining patients initially underwent endoscopic follow-up with repeat biopsy (n = 322), endoscopic resection (n = 129), or surgery (n = 6) for definite diagnoses or treatment. Of patients who underwent repeat biopsy, 28 (8.7%) showed recurrent gastric IND. These patients underwent additional procedures for definite diagnoses. Of 232 patients with non-neoplastic lesions in repeat biopsy, 131 cases were identified after 2 years of follow-up endoscopy. Additional neoplastic lesions were identified in seven cases (four LGD and three adenocarcinomas), but no lesions were found at previous gastric IND sites. The final histological results included adenocarcinomas (n = 128, 28%), HGD (n = 21, 4.6%), LGD (n = 31, 6.8%), and non-neoplasms (n = 277, 60.6%) (Fig. 2).

Clinical outcomes in patients with gastric lesions indefinite for dysplasia (IND) diagnosed by endoscopic forceps biopsy. HGD, mucosal high-grade neoplasia/dysplasia; LDG, low-grade neoplasia/dysplasia.
Comparison of the clinicopathological characteristics between revised Vienna category 1 to 3 and category 4 to 5 lesions
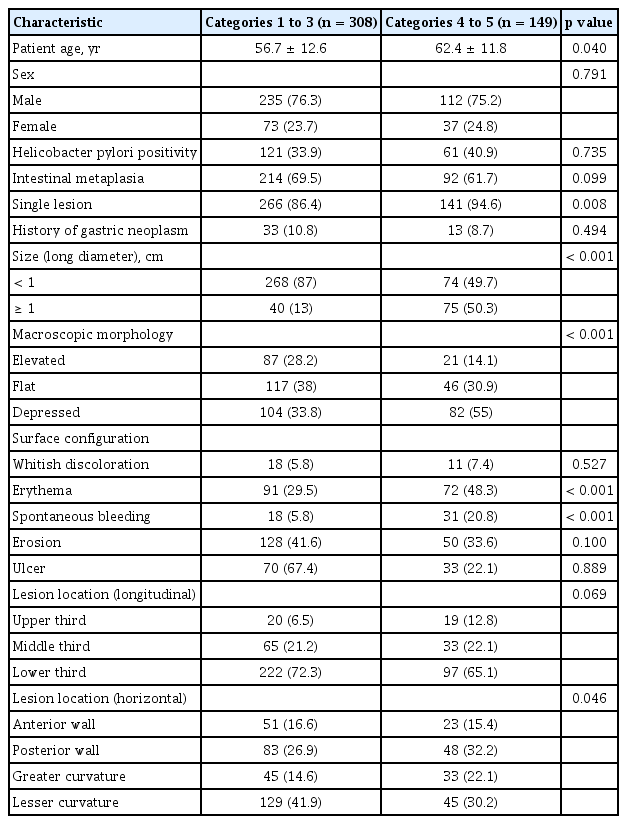
The baseline clinicopathological and endoscopic characteristics of the revised Vienna category 1 to 3 and category 4 to 5 groups are shown in Table 1. Mean age was significantly higher in the category 4 to 5 group than in the category 1 to 3 group (56.7 vs. 62.4, p = 0.04). Initial endoscopic findings showed that patients in the category 4 to 5 group had larger lesions of ≥ 10 mm (13% vs. 50.3%, p < 0.001) and a higher rate of single lesions (86.4% vs. 94.6%, p = 0.008) than those in the category 1 to 3 group. The rate of depressed lesions was significantly higher in the category 4 to 5 group than in the category 1 to 3 group (33.8% vs. 55%, p < 0.001). The surface configuration of lesions differed significantly in the incidence of surface erythema (29.5% vs. 48.3%, p < 0.001) and in the rate of spontaneous bleeding (5.8% vs. 20.8%, p < 0.001) between the category 4 to 5 and category 1 to 3 groups. PW and GC locations of the lesions were significantly more prevalent in the category 4 to 5 group. However, no significant differences in the rates of H. pylori positivity, intestinal metaplasia, and history of gastric neoplasm were found between the category 1 to 3 and category 4 to 5 groups.
Multivariate logistic regression analysis for the presence of Vienna category 4 to 5 lesions in gastric IND patients
Multivariate analysis showed that surface erythema (OR, 2.804; 95% CI, 1.741 to 4.516; p < 0.001), spontaneous bleeding (OR, 2.618; 95% CI, 1.298 to 5.279; p = 0.007), lesion diameter ≥ 1 cm (OR, 5.762; 95% CI, 3.459 to 9.597; p < 0.001), and depressed morphology (OR, 2.183; 95% CI, 1.155 to 4.124; p = 0.016) were significantly associated with a final diagnosis of Vienna category 4 to 5 lesions (Table 2).

Logistic regression analyses of endoscopic predictors of revised Vienna category 4 to 5 lesions in patients with gastric lesions indefinite for dysplasia diagnosed by endoscopic forceps biopsy
We evaluated the combined effects of independent risk factors (surface erythema, spontaneous bleeding, lesion diameter ≥ 1 cm, and depressed morphology) identified in the multivariate analysis on the presence of Vienna category 4 to 5 lesions (Fig. 3). ORs associated with 1, 2, and ≥ 3 risk factors were 1.596, 7.131, and 34.86, respectively, and the presence of ≥ 2 risk factors were significantly associated with Vienna category 4 to 5 lesions (Table 3).
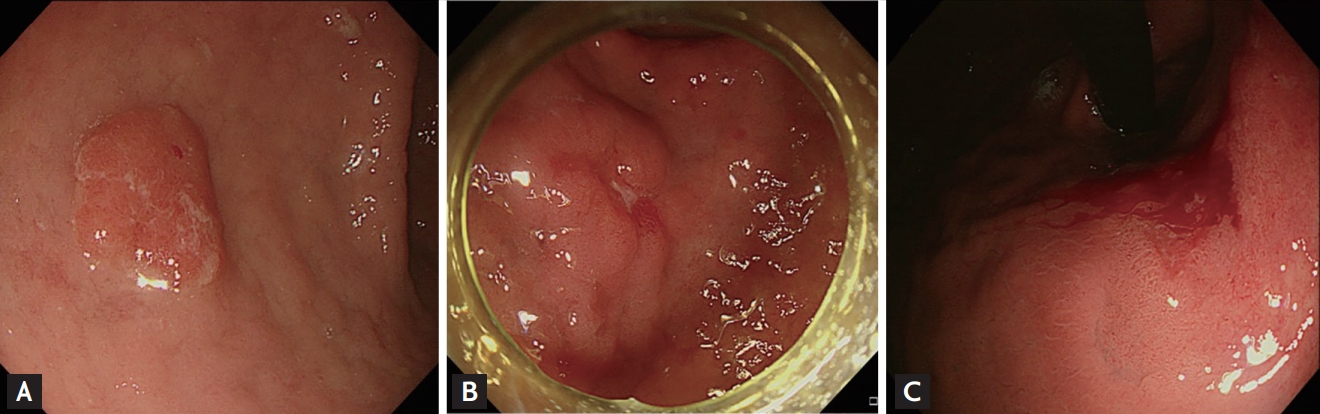
Endoscopic images of gastric lesions diagnosed as indefinite for dysplasia with risk factor relating to the presence of Vienna category 4 to 5. (A) 1 Risk factor: surface erythema. (B) 2 Risk factors: depressed lesion with surface erythema. (C) 3 Risk factors: lesion diameter ≥ 1 cm with surface erythema and spontaneous bleeding.
Clinical outcomes and adverse events of endoscopic resection as the first diagnostic option in gastric IND patients
Of the 457 patients with gastric IND, 129 initially underwent endoscopic resection as a part of their diagnostic and therapeutic management. After endoscopic resection, final pathological diagnoses were adenocarcinoma (n = 66, 51.2%), HGD (n = 15, 11.6%), LGD (n = 17, 13.2%), and non-neoplastic lesion (n = 31, 24%). The rate of en bloc resection was 95.9%, and that of piecemeal resection was 4.1%. Complete resection rate was 85.7%. Delayed bleeding after endoscopic resection occurred in 13 patients (10.1%), and these patients were successfully managed using endoscopic hemostatic techniques (epinephrine injection, hemoclips, and argon plasma coagulation). No patient experienced procedure-related perforation.
DISCUSSION
Few studies regarding the clinical outcomes of IND lesions in the upper gastrointestinal tract have been published to date. In a United States study, the annual incidence of esophageal adenocarcinoma and HGD in patients with Barrett’s esophagus and IND lesion was 0.8% [14]. In previous studies investigating the risk of neoplastic progression in gastric IND patients, the reported percentages of IND patients who developed gastric neoplasms varied from 26% to 47% [8,15,16]. In a recent study by Kim et al. [9], 92% (17/22) of gastric IND lesions were found to be malignant or premalignant. In the present study, 39.8% (182/457) of lesions were malignant or premalignant (128 adenocarcinomas and 54 dysplastic lesions), and the rate of Vienna category 4 to 5 lesions that could be treated with endoscopic resection was 32.6% (149/457). These findings show that the rate of definite neoplastic lesions was higher than generally expected. Endoscopists should thus consider the risk of neoplastic lesions in the management of gastric IND patients.
Therapeutic guidelines for gastric IND confirmed by EFB have not yet been established. The Vienna guidelines suggest that endoscopic surveillance with endoscopic re-biopsy is appropriate [3]. Although EFB is the gold standard for accurate diagnosis of suspected neoplastic lesions, forceps biopsy specimens do not always reflect the predominant histopathology of the lesion. A considerable discrepancy is reported between histological diagnoses made by EFB and diagnoses made with subsequent resection specimens in patients with gastric neoplasms. Recent reports indicate that 19% of the cases of LGD diagnosed by EFB may progress to HGD, or even adenocarcinoma, after endoscopic resection [5,6]. In a previous study performed in our hospital, the overall histological discrepancy rate between EFB and endoscopic resection specimens was 44.9% [4].
Histologic discrepancies between EFB and endoscopic resection might have resulted from the use of a small number of biopsy samples as well as the inhomogeneity of neoplastic lesions. In this study, the mean number of biopsy samples was significantly smaller (3.26 vs. 4.7, p = 0.001) at the time of the initial endoscopy than that at the time of the final diagnostic endoscopy. Previous studies have shown that an adequate number of biopsy samples should be obtained to improve diagnostic accuracy. Lal et al. [17] showed that diagnostic rate increased to 97.9% when four specimens were obtained and to 100% when six were obtained. However, if the lesion is endoscopically resectable, repeated or aggressive biopsy increases the rate of risk of adverse events for resection because of fibrosis and ulcers that develop following biopsy [18]. Thus, determination of the adequate number of biopsies can be difficult.
Because a simple follow-up strategy could lead to cases of missed adenocarcinomas owing to poor patient compliance or sampling errors with EFB, endoscopists may have difficulty determining an appropriate management strategy for gastric IND patients. In a United Kingdom study evaluating the rate of missed diagnoses in patients with upper gastrointestinal cancers, sampling errors and follow-up delay accounted for 25% and 9% of definitely missed diagnoses, respectively [19]. Cho et al. [20] reported that follow-up endoscopy was not performed in nine patients (36%) with gastric IND on index endoscopy. Therefore, clinicians cannot definitively rule out neoplastic lesions in gastric IND patients by endoscopic morphology, even if repeated forceps biopsies show non-neoplastic lesions. Some researchers suggest that more active management strategies, such as endoscopic resection, rather than follow-up endoscopic biopsy, should be considered for gastric IND patients for accurate diagnosis and complete treatment [8,15]. However, it is necessary to determine which patients with gastric IND diagnosed by EFB should consider endoscopic resection.
According to the Vienna classification, endoscopic or surgical resection is strongly recommended for category 4 to 5 lesions such as HGD and early gastric cancer, while therapeutic guidelines for category 3 lesions have not yet been established [3]. Understanding endoscopic characteristics of category 4 to 5 lesions is therefore important. Previous large studies identified endoscopic predictive factors of category 4 to 5 lesions in LGD patients. Cho et al. [6] reported that endoscopic resection can be recommended if a low-grade dysplastic lesion has at least one of the following traits: depressed morphology, surface erythema, or size of ≥ 1 cm. Kim et al. [5] suggested that endoscopic resection should be performed for gastric low-grade dysplastic lesions ≥ 2 cm and without whitish discoloration. Kim et al. [12] suggested that endoscopic resection should be performed if gastric low-grade dysplastic lesions have at least one of the following risk factors: surface erythema and depressed morphology regardless of size, or size of > 2 cm regardless of abnormal surface configuration. In this study, multiple factors such as size (lesion diameter ≥ 1 cm), spontaneous bleeding, surface color (erythema), and depressed morphology were significant predictive factors associated with the presence of category 4 to 5 lesions in patients diagnosed with EFB. These findings are similar to results for endoscopic factors for upgraded diagnosis in the LGD. Furthermore, we found that the effects of ORs in the presence of ≥ 2 risk factors (lesion diameter ≥ 1 cm, spontaneous bleeding, depressed morphology, and surface erythema), compared to those in the absence of risk factors or the presence of only 1 risk factor, were combined. Therefore, gastric IND patients with no or only one risk factor are less likely to develop HGD or invasive carcinoma. In clinical practice, endoscopic resection is recommended when ≥ 2 risk factors are present. However, an endoscopic follow-up strategy is recommended for such cases.
Endoscopic resection techniques including endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) were developed for minimally invasive organ-sparing endoscopic removal of benign and early malignant lesions in the gastrointestinal tract [21]. A study of endoscopic therapy outcomes in 1,832 Japanese patients with early gastric cancer treated with EMR and ESD showed complete resection in 73.9% of the patients and a combined adverse event rate of 1.9% (delayed bleeding, 1.4%; perforation, 0.5%) [22]. In a recent Korean study of ESD for 1000 early gastric neoplasms, a complete resection rate of 87% was achieved with a low rate of adverse events (delayed bleeding, 15.6%; perforation, 1.2%) [23].
In the present study, we evaluated clinical outcomes and adverse events of endoscopic resection as the first diagnostic option in gastric IND patients. The rate of endoscopic resection as the first diagnostic option for gastric IND was high, with 96% and 86% rates of en bloc resection and complete resection, respectively. An acceptable rate of adverse events (delayed bleeding, 10%; perforation, 0%) was reported. All cases of delayed bleeding after endoscopic resection were successfully managed by endoscopic hemostasis, and fatal procedure-related adverse events did not occur.
Our study has several limitations. First, we did not follow all patients diagnosed with non-neoplasms on the first re-biopsy. Final diagnoses in these cases could be inadequate. We minimized the possibility of misclassification by performing endoscopy in most of these patients in our hospital within two years, consistent with guidelines provided by the current Korean National Gastric Cancer Screening Program. Second, this study included pathologic data obtained from a single institute. Therefore, the possibility for selection bias exists. Further prospective multicenter studies are needed to thoroughly evaluate risk factors for gastric neoplasms and the clinical utility of endoscopic resection in patients with gastric IND.
In conclusion, this study suggests that precautions should be taken in the management of gastric IND patients, especially when risk factors, including surface erythema, spontaneous bleeding, lesion size ≥ 1 cm, and depressed morphology are present. Considering the combined effects of risk factors on the presence of HGD or adenocarcinomas, endoscopic resection should be recommended if at least two risk factors are present in a gastric IND patient.
KEY MESSAGE
1. Surface erythema, spontaneous bleeding, lesion diameter ≥ 1 cm, and depressed morphology were significantly associated with a final diagnosis of Vienna category 4 to 5 lesions.
2. The presence of ≥ 2 risk factors was significantly associated with Vienna category 4 to 5 lesions.
3. Considering the combined effects of risk factors on the presence of revised Vienna category 4 and 5 lesions, endoscopic resection should be recommended if two or more risk factors are present in gastric indefinite for dysplasia patients.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This work was supported by the Soonchunhyang University Research Fund.