Clinical features of Korean elderly patients with antineutrophil cytoplasmic antibody-associated vasculitis
Article information
Abstract
Background/Aims
We compared the clinical and laboratory data between elderly and non-elderly patients with antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) at diagnosis; further, we investigated the predictors at diagnosis for all-cause mortality and end-stage renal disease (ESRD) occurrence during follow-up in Korean elderly patients with AAV.
Methods
We reviewed the medical records of 191 AAV patients regarding clinical manifestations and laboratory results at diagnosis and during follow-up. The follow-up duration was defined as the period from diagnosis to death for deceased patients or to the time of dialysis for ESRD patients, or to the last visit. Elderly (n = 67) and non-elderly (n = 124) patients were grouped based on an age threshold of 65 years.
Results
At diagnosis, elderly patients exhibited higher median Birmingham Vasculitis Activity Score (BVAS) and higher frequencies of ANCA positivity and pulmonary manifestations than non-elderly patients. Furthermore, elderly patients exhibited increased median white blood cell count, blood urea nitrogen (BUN), alkaline phosphatase, erythrocyte sedimentation rate, and C-reactive protein and decreased median hemoglobin. However, there were no significant differences in all-cause mortality and ESRD occurrence between elderly and non-elderly patients. Meanwhile, elderly patients exhibited lower cumulative patients’ and ESRD-free survival rates than non-elderly patients. In the multivariable Cox hazards model, BUN, creatinine and serum albumin at diagnosis were independent predictors for ESRD occurrence, whereas there were no independent predictors at diagnosis for all-cause mortality.
Conclusions
Elderly AAV patients exhibited substantially higher rates of all-cause mortality and ESRD occurrence during follow-up compared than non-elderly AAV patients.
INTRODUCTION
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a group of systemic vasculitides involving small vessels. On the basis of the 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides (the 2012 definitions), AAV is defined as vasculitis predominantly affecting small vessels such as small intraparenchymal arteries, arterioles, capillaries, venules, and occasionally medium-sized arteries and veins [1]. AAV can be further classified into three variants such as microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA) and eosinophilic granulomatosis with polyangiitis (EGPA) based on clinical manifestations and histological features such as necrotizing vasculitis, granulomas and eosinophilic infiltration [1,2]. Compared to other vasculitis, particularly Takayasu arteritis or immunoglobulin A vasculitis (Henoch-Schonlein), AAV tends to occur in elderly people. Both MPA and GPA mainly occur in 60 to 70-year-old persons, whereas, EGPA often occurs in 40 to 60-year-old persons [3].
To date, there have been several studies comparing the clinical features and outcomes of young and elderly AAV patients: Early classification before advanced chronic kidney disease (CKD) and the use of immunosuppressive drugs improved the prognosis of AAV [4]; moreover, ESRD occurred more frequently in patients who were not treated with immunosuppressive drugs at 1 year after diagnosis. However, the rate of all-cause mortality was not different between the two groups [5]; elderly AAV patients exhibited higher incidence of both all-cause mortality and ESRD occurrence as well as the highest mortality rate within the first 6 months [6].
To the best of our knowledge, however, there is no report yet on the comparative analysis of the clinical and laboratory features and the incidence of all-cause mortality and ESRD occurrence in Korean elderly patients with AAV. Hence, in this study, we compared the clinical and laboratory features of elderly and non-elderly AAV patients at diagnosis and investigated the predictors at diagnosis for all-cause mortality and ESRD occurrence during follow-up in Korean elderly patients with AAV.
METHODS
Patient population
We reviewed the medical records of 191 AAV patients according to the following inclusion and exclusion criteria: (1) patients should have been first classified as AAV at the Division of Rheumatology, Department of Internal Medicine, Yonsei University College of Medicine, Severance Hospital, between October 2000 and October 2018; (2) patients should have well-documented medical records to confirm their classification as MPA, GPA or EGPA and to assess both clinical manifestations at diagnosis and death during follow-up, and calculate Birmingham Vasculitis Activity Score (BVAS) and five factor score (FFS) at diagnosis. Because BVAS for GPA has a different weight-system from BVAS, we evenly applied BVAS to GPA to unify the scoring system [7-9]; (3) patients should meet the American College of Rheumatology 1990 criteria and should be classified by the 2007 European Medicines Agency algorithm and the 2012 definitions [1,2]; (4) patients should have the results of perinuclear (P)-ANCA and cytoplasmic (C)-ANCA or myeloperoxidase (MPO)-ANCA and proteinase 3 (PR3)-ANCA. P-ANCA and C-ANCA were detected using an immunofluorescence assay and the titres of MPO-ANCA and PR3-ANCA were measured using an enzyme-linked immunosorbent assay [10]; (5) patients should have been followed up for 3 months or more; (6) patients should not have any serious medical conditions other than AAV, by which AAV activity and severity might be confused. Comorbidities at diagnosis or during follow-up were identified using the 10th revised International Classification Diseases (ICD-10) and immunosuppressive drugs administered for AAV were verified by the Korean Drug Utilization Review system. We collated a list of 224 AAV patients, who had been classified as AAV and had AAV as the primary diagnosis from October 2000, via the Clinical Data Repository System (CDRS) in November 2016. Of 224 AAV patients, 127 AAV patients fulfilled the inclusion criteria. In addition, from November 2016 to 3 months before the entry of this study, 74 patients were newly classified as AAV and added to this study population. Finally, 191 AAV patients were included in this study. This study was approved by the Institutional Review Board of Severance Hospital (IRB No. 4-2017-0673), which waived the need for written informed consent from the patients, as this was a retrospective study.
Clinical and laboratory data at diagnosis
At the time of AAV diagnosis, age and sex were compiled as demographic data. We calculated BVAS and FFS at diagnosis as AAV-specific indices. Clinical manifestations at diagnosis were recorded based on the items of BVAS and FFS: general, cutaneous, muco-membranous/ocular, ear nose throat, pulmonary, cardiovascular, gastrointestinal, renal, and nervous manifestations [7,9]. Routine laboratory data included positive ANCA types, white blood cell and platelet count, hemoglobin, fasting glucose, blood urea nitrogen (BUN), creatinine, total serum protein, serum albumin, alkaline phosphatase (ALP), aspartate transaminase (AST), alanine transaminase (ALT), total bilirubin, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP). At diagnosis, the results of tests for autoantibodies were also reviewed to exclude autoimmune diseases other than AAV.
Data during follow-up
During follow-up, all-cause mortality and ESRD were considered dependent variables in the prognosis analysis. In this study, patients, who received renal replacement therapy before diagnosis, were not counted as ESRD during follow-up. The follow-up duration based on either all-cause mortality or ESRD was defined as the period between the date of the diagnosis of AAV and the date of the last visit for surviving patients or those without renal replacement. In the case of deceased patients, the follow-up duration based on all-cause mortality was defined as the period between the diagnosis of AAV and the time of death. For patients who had ESRD, the follow-up duration for ESRD was defined as the period starting from the diagnosis of AAV until the initiation of renal replacement therapy. Comorbidities during follow-up included CKD (stage 3 to 5), diabetes mellitus, hypertension, interstitial lung disease (ILD), diffuse alveolar hemorrhage, ischemic heart disease, and cerebrovascular accident [3]. We also reviewed immunosuppressive drugs for AAV such as glucocorticoid, cyclophosphamide, rituximab, azathioprine, mycophenolate mofetil, tacrolimus, methotrexate, and plasma exchange.
Definition of an elderly AAV patient and severe AAV
The median age at diagnosis of study population (n = 191) was 56.1 years with an interquartile range (IQR) of 21.0. We chose 65 years at diagnosis rather than 75 years could be a better cut-off age for elderly AAV patients. When we defined an elderly AAV patient as a patient aged 65 years and above at the time of diagnosis [9], 124 patients comprised the non-elderly AAV patient-group and 67 patients comprised the elderly AAV patient-group. Additionally, we classified AAV patients aged 65 years or below at diagnosis as non-elderly AAV patients. We stratified AAV patients into three groups based on the tertile of BVAS and defined the lower limit of the highest tertile as the cut-off for severe AAV in the study (BVAS at diagnosis ≥ 18).
Statistical analysis
All statistical analyses were conducted using IBM SPSS Statistics software version 23 for Windows (IBM Corp., Armonk, NY, USA). Continuous variables were expressed as a median and IQR, and categorical variables were expressed as number and percentage. We divided AAV patients into the two groups using the age of 65 years and above or below as the criterion and compared variables between two groups. Significant differences in categorical variables between the two groups were analyzed using the chi-square and Fisher’s exact tests. Significant differences in continuous variables between the two groups were compared using the Mann-Whitney U test. Comparison of the cumulative patients’ and ESRD-free survival rate between the two groups were analyzed by the Kaplan-Meier survival analysis. In 67 patients with age ≥ 65 years, the multivariable Cox hazard model using variables with p value less than 0.05 and clinically relevant the hazard ratio (HR) in univariable Cox hazard model was conducted to appropriately obtain HRs and determine the predictors for all-cause mortality and ESRD occurrence during the considerable follow-up duration. The p values less than 0.05 were considered statistically significant.
RESULTS
Comparison of variables at diagnosis of elderly and non-elderly AAV patients based on an age cut-off of 65 years
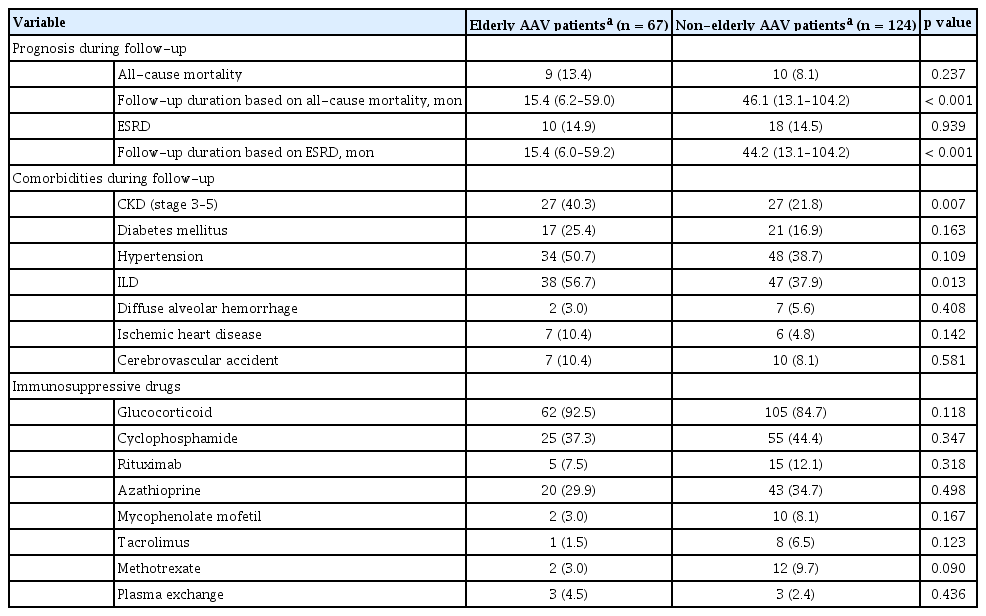
The median ages of elderly and non-elderly AAV patients were 71.0 and 50.0 years, respectively. The proportion of each AAV variant was evenly distributed in the two groups. MPO-ANCA (or P-ANCA) was detected more often in elderly AAV patients, whereas ANCA negative was observed more frequently in non-elderly AAV patients. Since FFS includes the age of 65 years or above as one item of five factors, the median FFS in elderly AAV patients at diagnosis was inevitably larger than that in non-elderly AAV patients. Meanwhile, age is not a sub-item of BVAS. Nonetheless, the median BVAS in elderly AAV patients at diagnosis was higher than that in non-elderly AAV patients (14.0 vs. 11.5, p = 0.037). Among clinical manifestations at diagnosis, only the frequency of pulmonary manifestation meaningfully differed between elderly and non-elderly AAV patients (73.1% vs. 48.4%, p = 0.001). Compared to non-elderly AAV patients, both elderly MPA patients and elderly GPA patients showed higher frequencies of pulmonary manifestation. However, elderly EGPA patients showed a frequency of pulmonary manifestation similar to that of non-elderly EGPA patients. In this study, we counted five types of lung lesions at diagnosis: ILD, ground glass opacity, lung nodules, cavitary lesion, and migratory lung infiltration. There were no significant differences in five types of pulmonary manifestation between elderly and non-elderly AAV patients. Among routine laboratory results at diagnosis, elderly AAV patients exhibited higher median white blood cell count, fasting glucose, BUN, ALP, ESR, and CRP than non-elderly AAV patients. In contrast, elderly AAV patients had lower median hemoglobin and serum albumin than non-elderly AAV patients (Table 1). There were no significant differences in the presence of autoantibodies between the two groups.
Comparison of variables during follow-up of elderly and non-elderly AAV patients based on an age cut-off of 65 years during follow-up
Nine of 67 elderly AAV patients (13.4%) died, whereas 10 of 124 non-elderly AAV patients (8.1%) died during follow-up based on all-cause mortality. In addition, ESRD occurred in 14.9% of elderly AAV patients and 14.5% of non-elderly AAV patients during follow-up based on ESRD. Among the comorbidities during follow-up, elderly AAV patients exhibited higher frequency of CKD than non-elderly AAV patients (40.3% vs. 21.8%, p = 0.007); however, patients in both groups showed similar frequencies of ESRD. Furthermore, the frequency of concurrent ILD in elderly AAV patients was much higher than that in non-elderly AAV patients (56.7% vs. 37.9%, p = 0.013). Glucocorticoid and immunosuppressive drugs were evenly administered to both groups (Table 2).
Comparison of the cumulative patients’ and ESRD-free survival rates of elderly and non-elderly AAV patients based on an age cut-off of 65 years
To closely examine the differences in the cumulative patients’ and ESRD-free survival rates between the two groups considering the different median follow-up durations, we conducted the Kaplan-Meier survival analysis. Elderly AAV patients exhibited lower cumulative patients’ survival rate than non-elderly AAV patients during follow-up based on all-cause mortality (p = 0.003) (Fig. 1A). Moreover, elderly AAV patients showed higher incidence of ESRD occurrence than non-elderly AAV patients during follow-up based on ESRD (p = 0.049) (Fig. 1B).
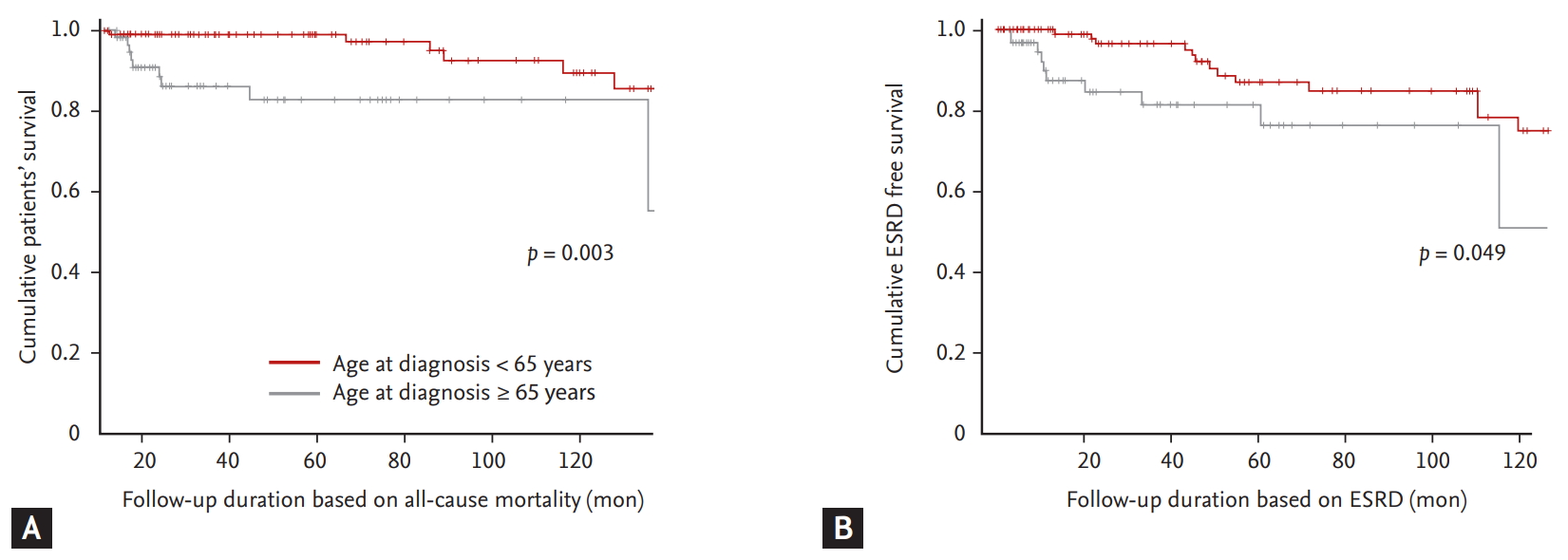
(A, B) Comparison of the cumulative survival rates based on the age cut-off of 65 years. Elderly antineutrophil cytoplasmic antibody-associated vasculitis (AAV) patients (aged ≥ 65 years) exhibited lower cumulative patients’ and end-stage renal disease (ESRD)-free survival rates than non-elderly AAV patients (aged < 65 years).
Predictors at diagnosis for all-cause mortality during follow-up in elderly AAV patients
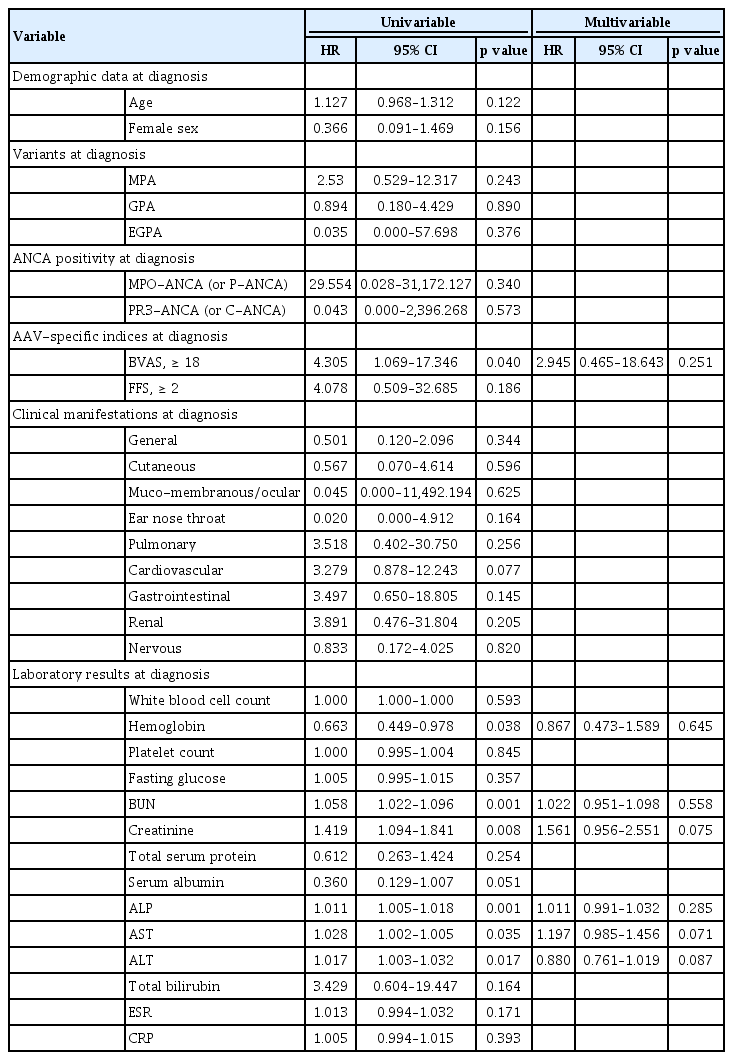
To obtain independent predictors at diagnosis for allcause mortality during follow-up, we conducted the univariable and multivariable Cox hazard model analyses. In the univariable Cox hazards model analysis, severe AAV (BVAS ≥ 18), hemoglobin, BUN, ALP, AST, and ALT at diagnosis were significantly associated with all-cause mortality in elderly AAV patients. However, no significant and independent predictors were found at diagnosis for all-cause mortality in the multivariable Cox hazards model analysis (Table 3).
Predictors at diagnosis for ESRD occurrence during follow-up in elderly AAV patients
We also analyzed the predictors for ESRD occurrence during follow-up using the univariable and multivariable Cox hazard model analysis. In the univariable Cox hazards model analysis, severe AAV, hemoglobin, BUN, creatinine, total serum protein, and serum albumin at diagnosis were significantly associated with all-cause mortality in elderly AAV patients. In general, total serum protein is inversely correlated with renal dysfunction. However, in the univariable analysis, total serum protein exhibited a positive HR, which is not clinically relevant. Thus, we did not include total serum protein in the multivariable analysis despite its statistical significance. In the multivariable Cox hazards model analysis, BUN (HR, 1.073; 95% confidence interval [CI], 1.016 to 1.133), creatinine (HR, 1.642; 95% CI, 1.132 to 2.383), and serum albumin (HR, 0.051; 95% CI, 0.006 to 0.444) were independent predictors at diagnosis for ESRD occurrence during follow-up based on ESRD in elderly AAV patients (Table 4).
DISCUSSION
In this study, we compared clinical and laboratory data between elderly and non-elderly AAV patients. We first investigated the predictors at diagnosis for all-cause mortality and ESRD occurrence during follow-up in elderly AAV patients in a monocentric retrospective cohort in Korea and obtained several interesting findings. In the cross-sectional comparative analysis of variables at AAV diagnosis, elderly AAV patients exhibited higher median BVAS and higher frequencies of MPO-ANCA (or P-ANCA) positivity, ANCA positivity and pulmonary manifestations than non-elderly AAV patients. Furthermore, elderly AAV patients exhibited higher median white blood cell count, BUN, ALP, ESR, and CRP and lower median hemoglobin than non-elderly AAV patients. In the cross-sectional comparative analysis of variables during follow-up, elderly AAV patients exhibited higher frequencies of CKD and ILD than non-elderly AAV patients. However, there were no significant differences in all-cause mortality and ESRD occurrence between elderly and non-elderly AAV patients.
With these results, we could make two assumptions. The first assumption is that highly active and severe AAV might occur more often in elderly people than in younger ones. The high median BVAS and increased frequencies of MPO-ANCA (or P-ANCA) and ANCA positivity in elderly AAV patients might support this assumption. Alternatively, even AAV, whose activity and severity are similar to those in non-elderly patients, could exaggerate the frequencies of AAV-specific manifestations due to low daily functionality in elderly AAV patients, leading to an increase in BVAS. The second assumption is that ageing itself might provoke enhanced autoimmunity and prolong autoinflammation. The detection rate of MPO-ANCA (or P-ANCA) is increased and the serological markers of chronic inflammation become apparent with age [5,11-14].
The Kaplan-Meier survival analysis revealed that elderly AAV patients exhibited lower cumulative patients’ and ESRD-free survival rates than non-elderly AAV patients, which were similar to the results of previous studies [4-6]. In addition, because the incidences of all-cause mortality and ESRD occurrence may gradually increase with age, if the age cut-off for elderly AAV patients is raised, would the difference be greater? The age cutoff for elderly AAV patients was set at 75 years and the cumulative patients’ and ESRD-free survival rates were compared between the two groups. Similarly, elderly AAV patients exhibited lower cumulative patients’ and ESRD-free survival rates than non-elderly AAV patients but the statistical power was much higher (Supplementary Fig. 1). However, as mentioned in the Methods section, the small number of elderly AAV patient could not warrant the statistical reliability. Therefore, we adopted the results of the comparative analysis of the cumulative survival rates between patients aged 65 years and above and those aged 65 years and below.
Regarding the predictors for ESRD occurrence during follow-up based on ESRD, BUN, creatinine and serum albumin at diagnosis were significant in the multivariable analysis. Because BUN and creatinine are variables directly associated with renal function, it could easily be accepted that the initial BUN and creatinine levels could predict the development of ESRD occurrence during AAV follow-up. Reduced serum albumin might be associated with persistent proteinuria, which can, in turn, accelerate renal function deterioration and finally provoke ESRD. Therefore, BUN, creatinine, and serum albumin at diagnosis could predict ESRD occurrence during follow-up based on ESRD regardless of the relationship between these variables and the concurrent activity of AAV [15].
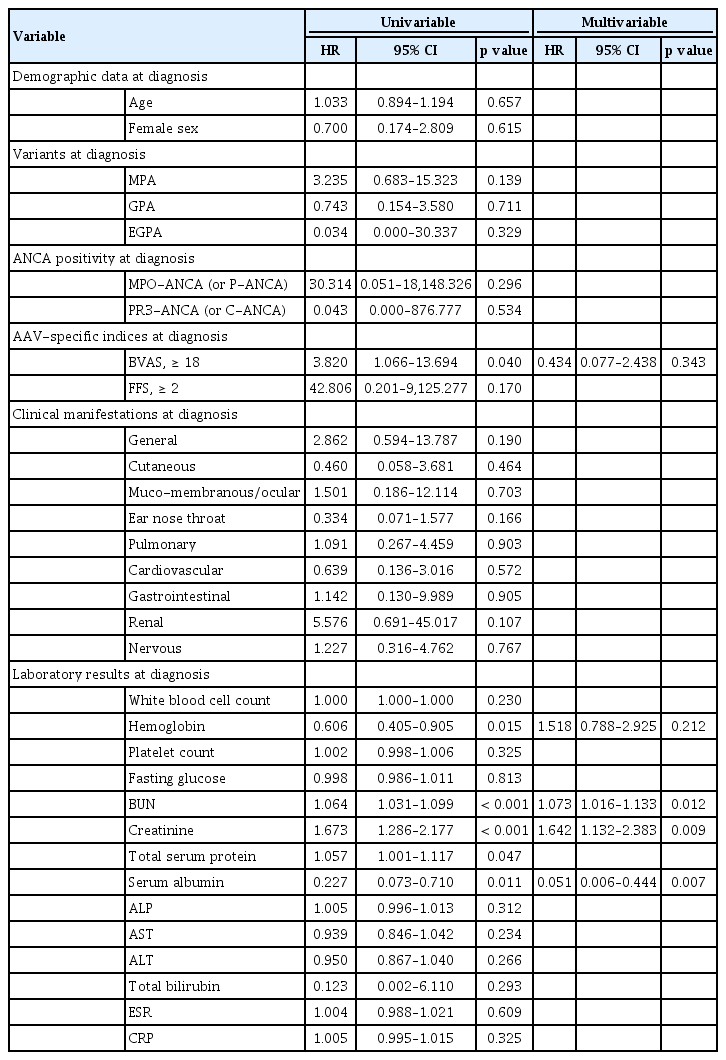
Additionally, we assessed predictors at diagnosis for all-cause mortality and ESRD in non-elderly AAV patients using the univariable and multivariable Cox hazards model analysis; then we compared the differences between independent predictors for all-cause mortality and ESRD between elderly AAV patients and non-elderly AAV patients. Both elderly and non-elderly AAV patients showed no independent predictor for all-cause mortality (Supplementary Table 1). On the other hand, elderly AAV patients exhibited three independent predictors, such as BUN, creatinine, and serum albumin, whereas non-elderly AAV patients showed four independent predictors, FFS ≥ 2, nervous manifestation, fasting glucose, and creatinine (Supplementary Table 2). Although it is difficult to determine the exact mechanism of the differences between the two groups, we hypothesize that the current activity of AAV at diagnosis may influence the progression to ESRD in non-elderly AAV patients, whereas advanced renal disease with low serum albumin at diagnosis may accelerate progression to ESRD in elderly AAV patients.
We believe that our study will contribute to understanding ethnic features in Korean elderly patients with AAV. We first demonstrated the cross-sectional differences in the clinical and laboratory features at diagnosis and prognosis as well as the comorbidities during follow-up between elderly and non-elderly AAV patients in Korea. Additionally, we provided information on the differences between the cumulative patients’ and ESRD-free survival rates based on the age cut-off of 65 years and the initial independent predictors for allcause mortality and ESRD occurrence during follow-up based on each outcome in Korean patients with AAV. However, our study also has several limitations. First, since we reviewed medical records of AAV patients retrospectively, we could not control the initial variables at AAV diagnosis and prevent missing data such as autoantibodies related to other autoimmune connective tissue diseases, despite searching the concurrent diseases of each patient by ICD-10. Additionally, we could not collate the conventional risk factors for all-cause mortality at the time of diagnosis, such as medical history and duration of diabetes mellitus, hypertension and dyslipidemia, smoking history, alcohol intake, and occupational factors. Concerning medications, the limitations of detailed content in the medical records discouraged the authors from calculating the cumulative dose of each medication administered owing to the retrospective study design and patients’ compliance and adherence to the oral immunosuppressive drugs. For this reason, we had to count the number of patients who received each drug. Second, the number of patients in this study was not large enough to provide more validated and stronger evidence on the features of Korean elderly patients with AAV. Third, the number of AAV patients aged 75 years and above was only 20; therefore, we could not clearly analyze the clinical and laboratory features at diagnosis and prognosis as well as the comorbidities during follow-up between patients aged 75 years and above and below. Given that previous studies conducted in Western countries selected the age for elderly AAV patients as 75 or 80 years, we could not compare the results of those previous studies with our results. Future multi-centric prospective studies with a large number of AAV patients, particularly more patients aged 75 years and above, will overcome these issues and provide more information on the features of Korean elderly patients with AAV.
In conclusion, elderly AAV patients exhibited substantially higher rates of all-cause mortality and ESRD occurrence during follow-up than non-elderly AAV patients. Furthermore, BUN, creatinine and serum albumin at diagnosis were independent predictors for ESRD during follow-up in Korean elderly patients with AAV.
KEY MESSAGE
1. Based on an age of 65 years, 67 of 191 antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) patients (35.1%) were classified as elderly patients.
2. Elderly AAV patients exhibited lower cumulative patients’ and end-stage renal disease (ESRD) free survival rates than non-elderly AAV patients.
3. Haemoglobin, blood urea nitrogen, creatinine, total protein and serum albumin at diagnosis were the independent predictors for ESRD in elderly AAV patients.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea (HI14C1324).
Supplementary Material
Supplementary Table 1.
Predictors at diagnosis for all-cause mortality during follow-up in non-elderly AAV patients (n = 124)
Supplementary Table 2.
Predictors at diagnosis for ESRD during follow-up in non-elderly AAV patients (n = 124)
Supplemental Figure 1.
(A, B) Comparison of the cumulative survival rates based on the age of 75 years. Elderly antineutrophil cytoplasmic antibody-associated vasculitis (AAV) patients (aged ≥ 75 years) exhibited the lower cumulative patients’ and end-stage renal disease (ESRD)-free survival rates than non-elderly AAV patients (aged < 75 years), similar to the comparative analysis based on the age of 65 years.