Impact of carotid atherosclerosis in CHA2DS2-VASc-based risk score on predicting ischemic stroke in patients with atrial fibrillation
Article information
Abstract
Background/Aims
Vascular disease is an established risk factor for stroke in patients with atrial fibrillation (AF), which is included in CHA2DS2-VASc score. However, the role of carotid atherosclerosis remains to be determined.
Methods
Three hundred-ten patients with AF who underwent carotid sonography were enrolled.
Results
During a median follow-up of 31 months, 18 events (5.8%) of stroke were identified. Patients with stroke had higher carotid intima-media thickness (CIMT) (1.16 ± 0.33 mm vs. 0.98 ± 0.25 mm, p = 0.017). CIMT was significantly increased according to the CHA2DS2-VASc score (p < 0.001) and it was correlated with left ventricular mass index and early diastolic mitral annular velocity (e’), a ratio of early transmitral flow velocity to e’ (E/e’) and pulmonary artery systolic pressure (all p < 0.05). Cox regression using multivariate models showed that carotid plaque was associated with the risk of stroke (hazard ratio, 3.748; 95% confidence interval [CI], 1.107 to 12.688; p = 0.034). C-statistics increased from 0.648 (95% CI, 0.538 to 0.757) to 0.716 (95% CI, 0.628 to 0.804) in the CHA2DS2-VASc score model after the addition of CIMT and carotid plaque as a vascular component (p = 0.013).
Conclusions
Increased CIMT and presence of carotid plaque are associated with a high risk of ischemic stroke, and CIMT is related to myocardial remodeling and diastolic dysfunction, suggesting that carotid atherosclerosis can improve risk prediction of stroke in patients with AF, when included under vascular disease in the CHA2DS2-VASc scoring system.
INTRODUCTION
Prevention of ischemic stroke and thromboembolism is a major therapeutic goal in patients with atrial fibrillation (AF) [1,2]. Vascular disease is a well-known risk factor for ischemic stroke in patients with AF, and is a component of risk stratification using CHA2DS2-VASc score [3]. The universal definition of vascular disease includes a history of myocardial infarction, peripheral arterial diseases or aortic plaque.
Carotid atherosclerosis, including carotid intima media thickness (CIMT) and presence of carotid plaque, is an independent risk factor for stroke in the general population [4-7]. Carotid atherosclerosis is also associated with higher AF incidence and may also contribute to the etiopathogenesis of AF [8-11]. However, whether carotid atherosclerosis is a major contributor to the development of ischemic stroke in patients with AF is unclear. If there is an association between carotid atherosclerosis and ischemic stroke, carotid atherosclerosis should be considered a vascular disease in CHA2DS2-VASc score. In addition, whether carotid atherosclerosis is the direct embolic source of ischemic stroke or a surrogate marker of systemic atherosclerosis and myocardial disease in patients with AF remains to be elucidated.
Hence, the aim of this study was to evaluate the association between carotid atherosclerosis and ischemic stroke. We also investigated the relation between carotid atherosclerosis and myocardial structure and function.
METHODS
Study population
Three hundred-ten patients with AF who underwent carotid sonography were retrospectively identified at a single tertiary institution. Baseline clinical variables before carotid sonography, including age, sex, body mass index, medical history and concomitant medical therapies, were assessed. This study was performed in accordance with the declaration of Helsinki and approved by the Institutional Review Board of Korea University Hospital (2018AN0053). Written informed consent was waived because the present study is based on retrospective analysis.
Outcome measurements
Primary endpoint included the incidence of ischemic stroke, myocardial infarction (MI), heart failure (HF), all-cause mortality, or a composite of endpoints (CE) including ischemic stroke, MI, HF, and all-cause mortality. Ischemic stroke was determined with clinical medical records and radiologic findings.
Carotid sonography
Carotid sonography was performed following the American Society of Echocardiography consensus statement [12]. Ultrasonographic images of both left and right carotid arteries were obtained at 1 cm proximal to the carotid bifurcation by cardiologists. A commercially available echocardiographic system (Vivid-I or E9, VingmedGeneral Electric, Horten, Norway) with a 14-MHz linear transducer was used [13].
CIMT was evaluated by high-resolution B-mode ultrasonography [14]. Intima-media interface lines was measured automatically using an automatic border detection program in Vivid system. The maximal CIMT was defined as the greatest intima-media thickness in either the left or right carotid artery extending from the bulb to 1 cm above the carotid sinus [6].
The presence of carotid plaque was defined as follows: (1) abnormal wall thickness (CIMT > 1.5 mm) or (2) a focal lesion measuring at least 0.5 mm or 50% of the surrounding CIMT value and protruding into the lumen.
Transthoracic echocardiography
Among patients in this cohort, 269 patients underwent two-dimensional and Doppler echocardiography within 3 months from the date of carotid sonography. Chamber quantification was performed using 2D echocardiography images, and left ventricular mass index (LVMI) was calculated using the formula recommended by the American Society of Echocardiography [15]. Mitral inflow velocity was obtained in the apical four-chamber view by pulsed-wave Doppler echocardiography during early filling (E) and late filling (A). The early diastolic mitral annular velocity (e’) of the septal mitral annulus was evaluated by tissue Doppler imaging. Pulmonary artery systolic pressure (PASP) was estimated using the peak velocity of tricuspid regurgitation [16].
Statistical analysis
Data were presented as mean ± SD for continuous variables or frequencies (percentages) for categorical variables. Clinical characteristics were compared according to the presence of carotid plaque using the Student’s t test or chi-square test. Mean values of CIMT based on incidence of ischemic stroke were compared using Wilcoxon rank sum test and CHA2DS2-VASc score was compared using analysis of variance test with contrast. The cumulative incidence rate of primary endpoints was calculated using the Kaplan-Meier method and the patients who were lost to follow-up were censored. The groups were compared by the log-rank test. A multivariate Cox regression analysis, adjusting age, sex, hypertension, diabetes mellitus, congestive heart failure, prior myocardial infarction, prior stroke, peripheral artery disease, chronic kidney disease, was performed to assess the impact of carotid plaque on primary endpoint. To obtain cut-point of CIMT to predict ischemic stroke, the indicator variable defined by the cut-point was added into multiple logistic regression model, including the CHA2DS2-VASc score. To evaluate whether carotid atherosclerosis improved the prediction of ischemic stroke, we included carotid plaque or CIMT as a vascular component of CHA2DS2-VASc score. The C-statistics was assessed for model discrimination, and each model was compared using DeLong test [17]. SPSS version 22.0 for Windows (IBM Co., Armonk, NY, USA) and R version 3.0.2 (R development core team, Vienna, Austria) were used to perform statistical analysis. A two-sided p value < 0.05 was considered statistically significant.
RESULTS
Baseline characteristics
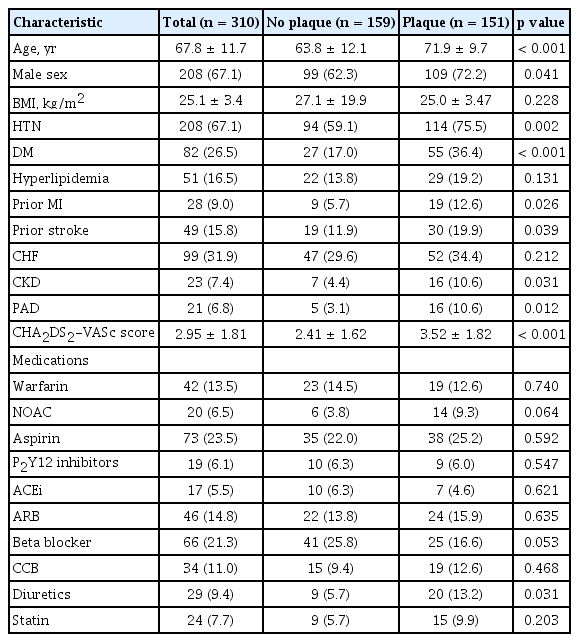
A total of 310 patients with AF underwent carotid sonography at the Korea University Hospital during the study period. The baseline characteristics of the patients are shown in Table 1. The characteristics were compared between patients with and without carotid plaque. In patients with carotid plaque, the mean age was greater and the proportion of males was higher compared with those without carotid plaque. Patients with carotid plaque showed an increased tendency for hypertension, diabetes mellitus, prior MI, prior stroke, HF, chronic kidney disease and peripheral artery disease. The CHA2DS2-VASc score was higher in patients with carotid plaque. The concomitant medications were not different between two groups except for diuretics.
CIMT and its relation
As shown in Fig. 1, CIMT increased in patients with high CHA2DS2-VASc score (0.9264 ± 0.2523 in 0 to 2; 1.038 ± 0.2343 in 3; 1.015 ± 0.2461 in 4 to 5; 1.163 ± 0.2756 in ≥ 6; p < 0.001). CIMT was significantly correlated with myocardial structural remodeling represented by LVMI (r = 0.159, p = 0.010) and myocardial diastolic dysfunction represented by septal e’ velocity, E/e’ ratio and PASP (e’: r = –0.215, p = 0.001; E/e’ ratio: r = 0.253, p < 0.001; PASP: r = 0.165; p = 0.012) (Fig. 2).
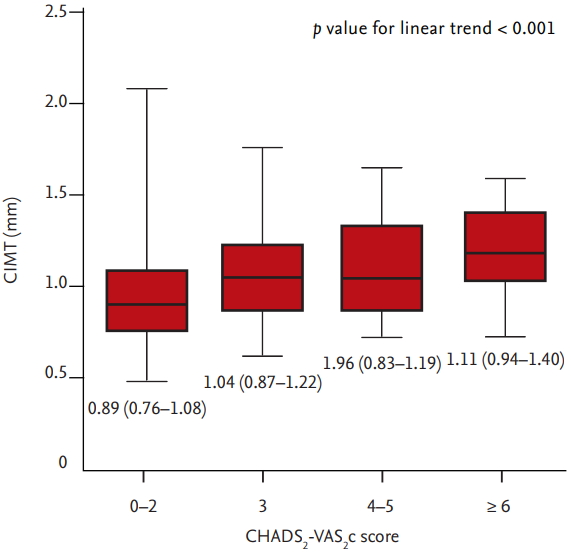
The relation between carotid intima media thickness (CIMT) and CHA2DS2-VASc score. CIMT increased in patients with high CHA2DS2-VASc score.
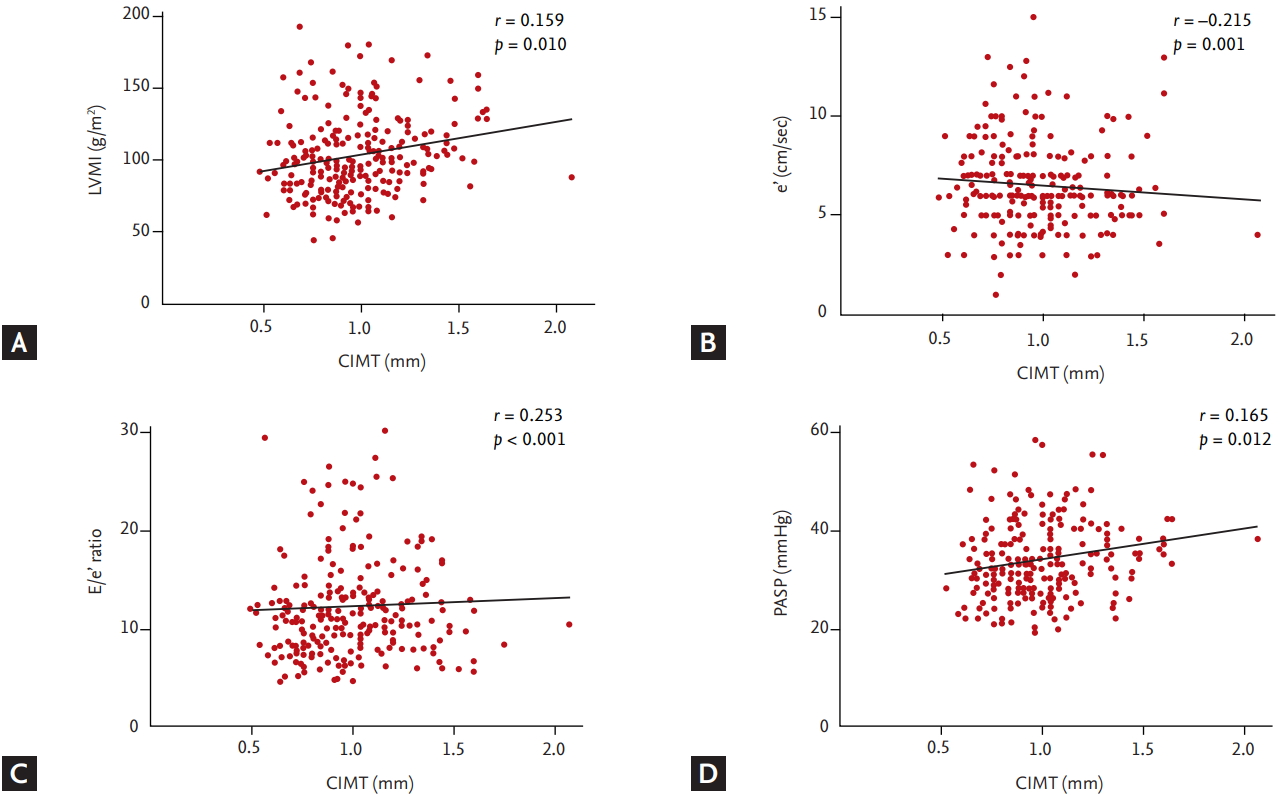
The relationship between carotid intima media thickness (CIMT) and echocardiographic parameters. (A) Left ventricular mass index (LVMI), (B) e’, (C) E/e’ ratio, and (D) pulmonary artery systolic pressure (PASP). CIMT was significantly correlated with myocardial structural remodeling represented by LVMI and myocardial diastolic dysfunction represented by septal e’ velocity, E/e’ ratio, and PASP. e’, early diastolic mitral annular velocity; E, early filling velocity of mitral inflow.
CIMT, carotid plaque and cardiovascular events
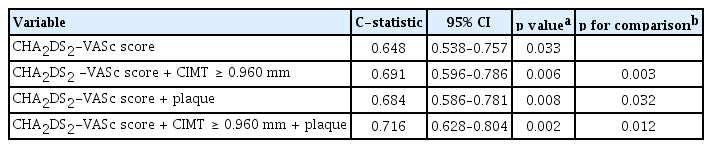
During a median follow-up of 31 months, 46 events (14.8%) of the primary endpoint were identified in patients with AF (MI, 6; stroke, 18; HF, 30; and all-cause mortality, 8). Mean and maximal CIMT in the left carotid artery were larger in patients with ischemic stroke compared to those without ischemic stroke, although their difference of right carotid artery was not statistically significant (left mean CIMT: 0.7610 ± 0.1801 mm vs. 0.8694 ± 0.1654 mm, p = 0.009; left maximal CIMT: 0.9236 ± 0.2467 mm vs. 1.064 ± 0.2109 mm, p = 0.010; right mean CIMT: 0.7454 ± 0.1727 mm vs. 0.8418 ± 0.2448 mm, p = 0.116; right maximal CIMT: 0.9000 ± 0.2308 mm vs. 1.057 ± 0.3691 mm, p = 0.084). Patients with ischemic stroke showed greater maximal CIMT (0.9830 ± 0.2503 mm vs. 1.161 ± 0.3281 mm, p = 0.017) and higher prevalence of carotid plaque compared with those without stroke (77.8% vs. 46.9%, p = 0.014) (Fig. 3). Cox regression analysis using multivariate models showed that carotid plaque was significantly associated with the incidence of ischemic stroke (hazard ratio [HR], 3.748; 95% confidence interval [CI], 1.107 to 12.688; p = 0.034), HF (HR, 2.725; 95% CI, 1.115 to 6.662; p = 0.028) and a CE (HR, 4.155; 95% CI, 1.945 to 8.872; p < 0.001) (Table 2). Kaplan-Meir curves showed that the cumulative incidence of stroke (p = 0.008), CE (p < 0.001), and HF (p = 0.006) were significantly higher in patients with carotid plaque compared with those without carotid plaque, but there was no difference in MI (Fig. 4). The cut-point of maximal CIMT to predict ischemic stroke was 0.960 mm in the multiple logistic regression model including the CHA2DS2-VASc score. The C-statistics increased from 0.648 (95% CI, 0.538 to 0.757) to 0.716 (95% CI, 0.628 to 0.804) in the CHA2DS2VASc score model after addition of CIMT and carotid plaque as vascular components (p = 0.013) (Table 3).
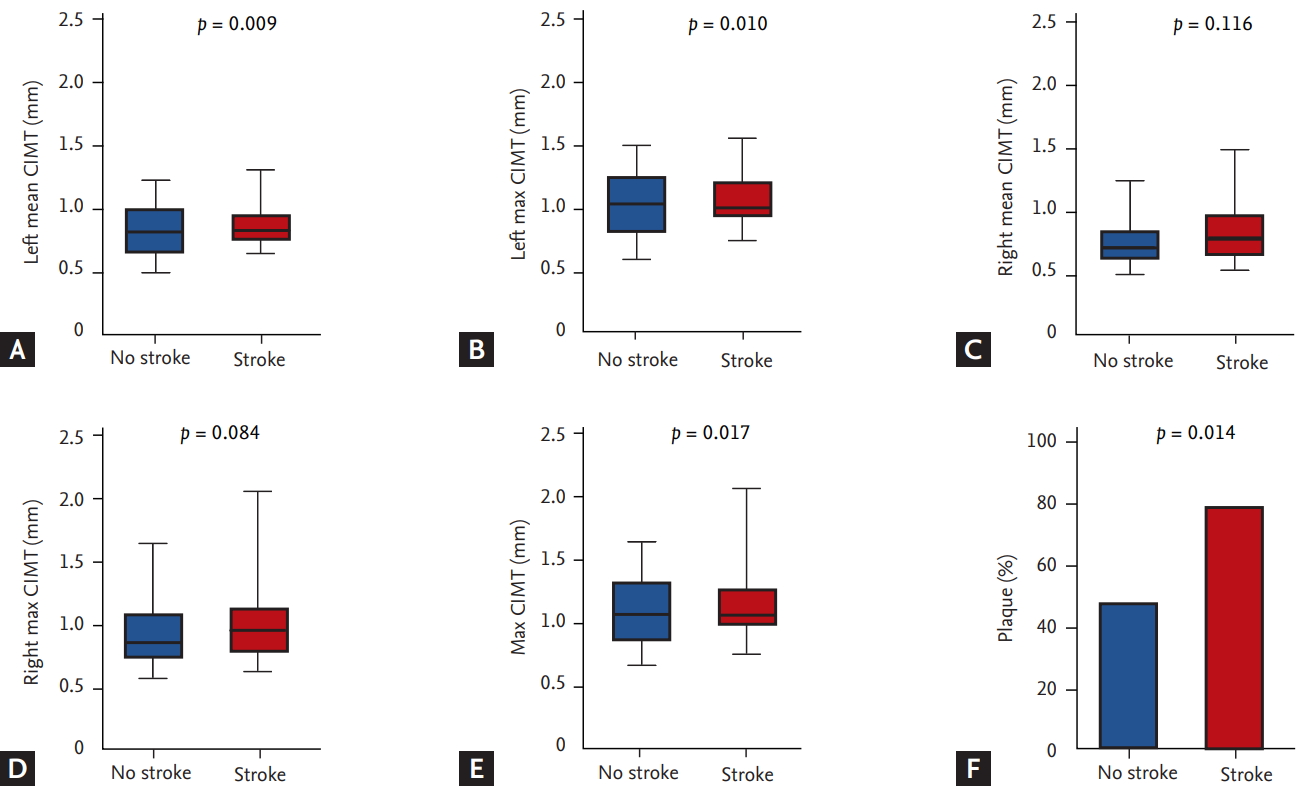
Carotid intima media thickness (CIMT) and the prevalence of carotid plaque according to presence of ischemic stroke. (A) Left mean CIMT, (B) left maximal CIMT, (C) right mean CIMT, (D) right maximal CIMT, (E) maximal CIMT, and (F) the prevalence of carotid plaque. The mean and maximal CIMT in the left and right carotid arteries and the prevalence of carotid plaque were significantly larger in patients with ischemic stroke compared with those without ischemic stroke.
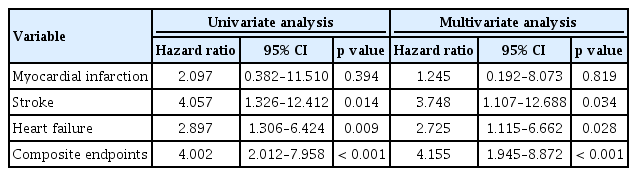
Cox regression analysis using univariate and multivariate models of association between carotid plaque and clinical events
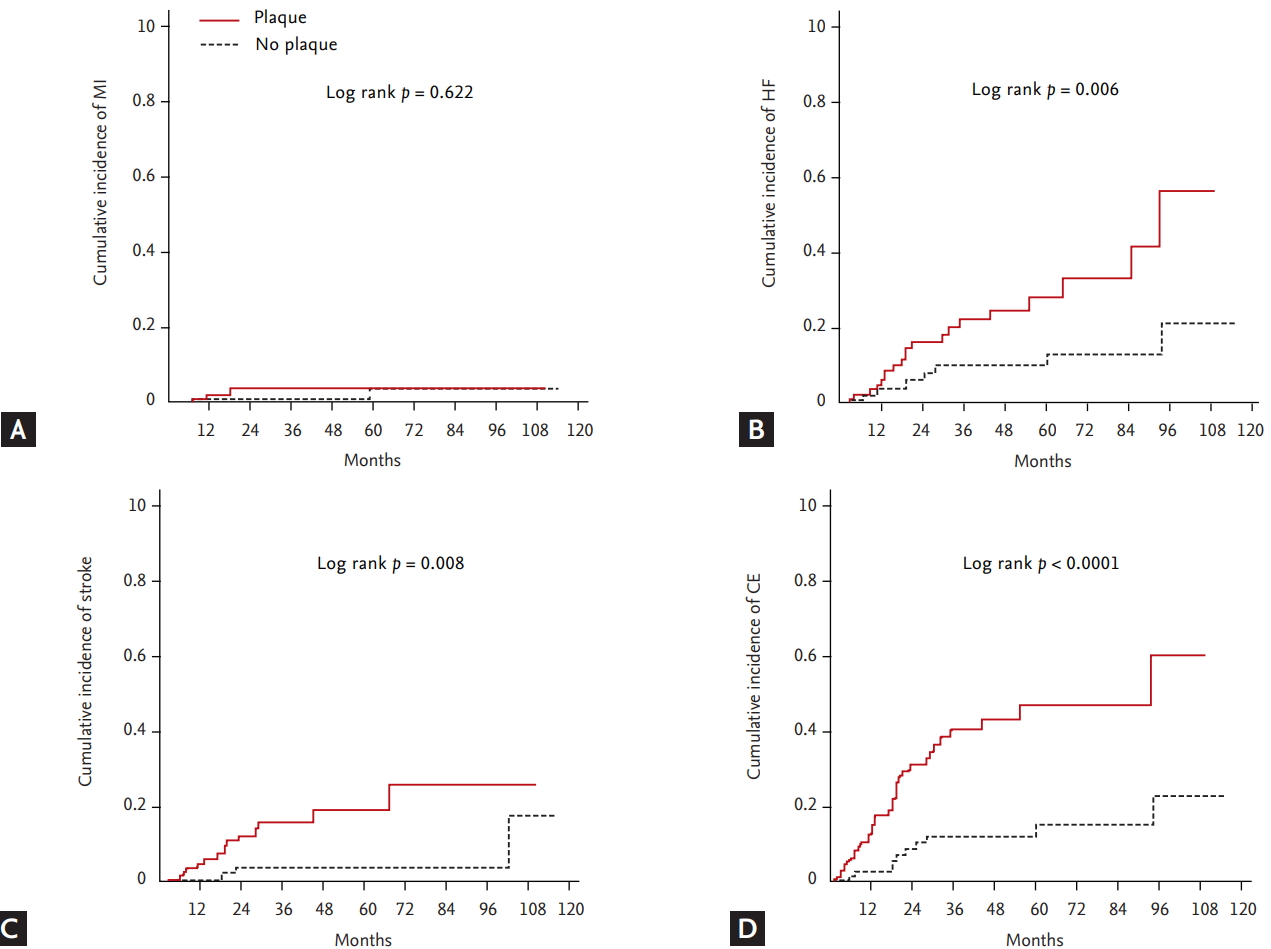
Kaplan-Meier curves of primary endpoints. (A) Myocardial infarction (MI), (B) heart failure (HF), (C) ischemic stroke, and (D) composite endpoint (CE) according to the presence of carotid plaque. The cumulative incidence of HF, stroke and CE were significantly higher in patients with carotid plaque compared with those without carotid plaque, but not MI.
DISCUSSION
In patients with AF, we demonstrated that: (1) carotid atherosclerosis was associated with a higher cardiovascular risk for ischemic stroke; (2) carotid atherosclerosis was impaired in patients with ischemic stroke more than in those without stroke; (3) CIMT showed significant correlation with CHA2DS2-VASc score, myocardial remodeling and diastolic dysfunction; (4) carotid plaque was an independent risk factor for ischemic stroke; and (5) CIMT and carotid plaque incrementally predicted the risk of ischemic stroke, suggesting that these risk factors might be associated with vascular disease in CHA2DS2VASc score. This is the first study to evaluate clinical impact of carotid atherosclerosis on ischemic stroke in an Asian population with AF. The association between carotid atherosclerosis and multiple cardiovascular risk factors and cardiac remodeling suggests that systemic atherosclerosis could be one mechanism for the development of ischemic stroke in AF patients.
We also investigated the relation between carotid atherosclerosis and myocardial structure and function in AF patients.
Carotid atherosclerosis and AF
Previous studies reported that vascular atherosclerosis was associated with AF [5,8,10,18,19]. The Rotterdam study, a cohort of 4,407 subjects without AF demonstrated the association between CIMT, carotid plaque and the risk of new onset AF, suggesting that patients with higher atherosclerotic burden tended to carry a higher risk of AF development [18]. Subclinical atherosclerosis may cause atrial tissue damage by reducing coronary blood supply to atrium, causing premature apoptosis of myocytes, fibrotic replacement and enhanced reentry process [20-22]. Myocardial ischemia and increased afterload following systemic atherosclerosis may trigger myocardial remodeling, myocardial diastolic dysfunction, increased left atrial filling pressure and left atrial enlargement [23-26]. Our study demonstrated that carotid atherosclerosis in AF patients was strongly correlated with myocardial remodeling (LVMI) and myocardial diastolic dysfunction (e’, E/e’ ratio and PASP), supporting the correlation between CIMT and myocardial dysfunction.
Carotid atherosclerosis and ischemic stroke in AF patients
The CHA2DS2-VASc scoring system only includes myocardial infarction, peripheral arterial disease, and aortic plaque but not carotid atherosclerosis as components of vascular disease [3]. However, diagnosis of aortic plaque is difficult in routine clinical practice. Carotid atherosclerosis is easily and non-invasively assessable by carotid sonography. It can be commonly used for risk stratification in patients with AF. Furthermore, carotid atherosclerosis haves anatomic and functional proximity to the cerebral vascular system compared to other vascular diseases. Recently, two prospective studies reported the effect of carotid atherosclerosis on ischemic stroke in AF patients [27,28]. The Atherosclerosis Risk in Communities (ARIC) study, a population-based study, which analyzed the association between carotid atherosclerosis and ischemic stroke in 784 AF patients showed 81 (11.2%) cases of ischemic stroke and CIMT, and carotid plaque increased C-statistic in addition to CHA2DS2-VASc score [27]. During a median follow-up of 36 months involving 2,017 patients with AF in the Atrial Fibrillation Registry for Ankle-brachial Index Prevalence Assessment: Collaborative Italian Study, addition of carotid plaque to conventional vascular disease was independently associated with ischemic stroke [28]. In our study, the association between carotid plaque and ischemic stroke was consistent with prospective studies. However, it is still not clear whether the association between carotid atherosclerosis and ischemic stroke resulted from thromboembolism or atherothrombosis [29]. Our study suggested that carotid atherosclerosis was a surrogate marker of systemic atherosclerosis and was not only a direct source of atherothrombosis, but was also related to myocardial remodeling as a source of thromboembolism.
Clinical implications
This study suggests that carotid atherosclerotic burden in patients with AF may be considered as a vascular disease in the CHA2DS2-VASc risk score. Especially, the addition of increased CIMT (defined as more than 0.960 mm) or carotid plaques significantly improved the predictive value of CHA2DS2-VASc risk score. Therefore, therapeutic implications associated with anticoagulants may be extended to patients with AF diagnosed with subclinical carotid atherosclerosis.
Study limitations
The present study has several limitations. First, although carotid atherosclerosis was associated with ischemic stroke, the impact of medications including antiplatelet agents or anticoagulants on ischemic stroke might not be significant in our study because of the small size number of patients enrolled and the different types of drugs prescribed in clinical practice. In this study, the proportion of patients with anticoagulation is relatively low compared to the CHA2DS2-VASc score. In Korea, the proportion of patients with anticoagulation was low. However, it significantly increased after change in health insurance coverage policy for new oral anticoagulants in July 2015 [30]. A large-scale prospective study is needed to elucidate medical interventions including effects of new oral anticoagulants for carotid atherosclerosis in patients with AF. Second, the pathophysiologic role of carotid atherosclerosis is still unclear although we showed the relation between carotid atherosclerosis and myocardial remodeling. Third, even though AF patients who underwent carotid sonography were enrolled, our results derived from a retrospective, single center experience could not be generalized to a broader population. However, key clinical risk factors were adjusted in the correlation between carotid plaque and clinical events. Finally, this study demonstrated that the addition of increased CIMT (defined as more than 0.960 mm) improved the predictive value of CHA2DS2VASc risk score. The absolute value of high CIMT needs to be validated based on a large-scale population study to include vascular component of CHA2DS2-VASc score.
In conclusion, increased CIMT and carotid plaque levels are associated with the risk of ischemic stroke. CIMT is also related to myocardial remodeling and diastolic dysfunction. These findings suggest that carotid atherosclerosis in patients with AF can be a surrogate marker of ischemic stroke or systemic atherosclerosis, and represent a prognostic factor improving the risk prediction of stroke, when incorporated into the vascular disease component of the CHA2DS2-VASc score.
KEY MESSAGE
1. Vascular disease is an established risk factor for stroke in patients with atrial fibrillation (AF), which is included in CHA2DS2-VASc score. However, the role of carotid atherosclerosis remains to be determined.
2. Increased higher carotid intima-media thickness and presence of carotid plaque are associated with a high risk of ischemic stroke.
3. Carotid atherosclerosis is a surrogate marker of systemic atherosclerosis and is not only a direct source of atherothrombosis, but is also related to myocardial remodeling and dysfunction as a source of thromboembolism.
4. Carotid atherosclerosis can improve risk prediction of stroke in patients with AF.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This work was supported by a Korea University Grant (Jong-Il Choi) and a grant of Korea University Anam Hospital (Jong-Il Choi) and in part by grants from Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (NRF-2015R1D1A1A02061859 to Jong-Il Choi) and the Ministry of Science, ICT & Future Planning (NRF-2012R1A1A1013260 to Jong-Il Choi).