Clinical implications of anti-thyroglobulin antibody measurement before surgery in thyroid cancer
Article information
Abstract
Thyroglobulin antibody (TgAb) is a class G immunoglobulin and a conventional marker for thyroid autoimmunity. From a clinical perspective, TgAb is less useful than thyroid peroxidase antibodies for predicting thyroid dysfunction. However, TgAb is found more frequently in differentiated thyroid cancer (DTC) and can interfere with thyroglobulin (Tg) measurements, which are used to monitor the recurrence or persistence of DTC. Recent studies suggested a small but consistent role for preoperative TgAb in predicting DTC in thyroid nodules, and in reflecting adverse tumor characteristics or prognosis, including lymph node metastasis, but this is still controversial. Postoperative TgAb can serve as a biomarker for remnant thyroid tissue, so follow-up measures of TgAb are useful for predicting cancer recurrence in DTC patients. Since high serum TgAb levels may also affect the fine needle aspiration washout Tg levels from suspicious lymph nodes of DTC patients, it is important to use caution when interpreting the washout Tg levels in patients who are positive for TgAb.
INTRODUCTION
Differentiated thyroid cancer (DTC) is the most common endocrine cancer, and its incidence has been increasing worldwide. DTC is found in 7% to 15% of thyroid nodules, and it has a good prognosis [1]. The most common type of DTC is papillary thyroid cancer (PTC), comprising more than 90% of all thyroid cancers, whereas follicular thyroid cancer is less common, comprising 7% to 8% of thyroid cancers in Korea [2,3]. Lobectomy or total thyroidectomy is generally the treatment of choice, except for some microcarcinomas. After thyroidectomy, thyroid hormone replacement and radioactive iodine (RI) for remnant ablation are administered [4].
Serum thyroglobulin (Tg) is the most sensitive established biomarker of DTC after total thyroidectomy and RI. Tg is produced only in normal thyroid tissue or in thyroid cancer tissue, so measuring Tg levels in the blood is useful for the early detection of recurrent or residual disease in DTC patients who have undergone total thyroidectomy and RI. Disease-free individuals have extremely low Tg levels.
Tg levels could be affected by the presence of thyroglobulin antibody (TgAb), which falsely lowers or elevates serum Tg levels [5,6]. Thus, current cancer management guidelines mandate that Tg testing should always include the measurement of TgAb. Generally, an elevated TgAb level alone rarely indicates any specific disease states or changes, except during the post-operative follow-up of DTC patients with positive TgAb levels. However, studies have shown that the clinical use of TgAb has the potential to improve patient care. In this article, we summarize the ways that TgAb is being used in a variety of clinical settings in the thyroid cancer field.
CHARACTERISTICS OF TgAb
There are many thyroid-related antibodies that target specific proteins within or around the thyroid follicular cells, including thyroid peroxidase (TPO), thyroid-stimulating hormone (TSH) receptor, Tg, and, rarely, carbonic anhydrase 2, megalin, triiodothyronine, thyroxine, sodium iodide symporter (NIS), and pendrin [7]. Thyroid-related antibodies are usually related to thyroid autoimmune diseases: TPO antibodies (TPOAb) and TgAb indicate Hashimoto’s thyroiditis (HT), and TSH receptor antibodies indicate Graves’ disease (GD).
TgAb was the first antibody found in patients with autoimmune thyroid disease (AITD) as described by Doniach and Roitt in 1956 [8,9]. Most TgAbs are classified as immunoglobulin G (IgG), though some are IgA. It is an intra-follicular antibody that can bind to immune cells and antigens with or without tissue destruction. Massive destruction of the thyroid gland induces structural changes in Tg, leading to antibody production against Tg. Major T-cell epitopes on Tg require iodination for recognition by autoreactive T-cells [10]. This explains the higher incidence of positive TgAb levels in patients with an excessive iodine intake [11]. However, high Tg levels in blood do not necessarily induce antibody production. The TgAb level depends on antigen exposure time [7]. There are nearly 40 Tg epitopes, of which only a few are immunogenic [12]. TgAb itself does not cause thyroid cell destruction, and it does not fix complement because the epitopes are too widely spaced to allow cross-linking [13].
TgAb AND DTC
Studies have suggested that TgAb plays a role in the relationship between autoimmune thyroiditis and thyroid cancer; most of these studies have been pathological studies, though some are serological studies [14-16]. However, it is unclear what roles the thyroid autoantibodies that indicate thyroiditis play in the pathogenesis of thyroid cancer [7].
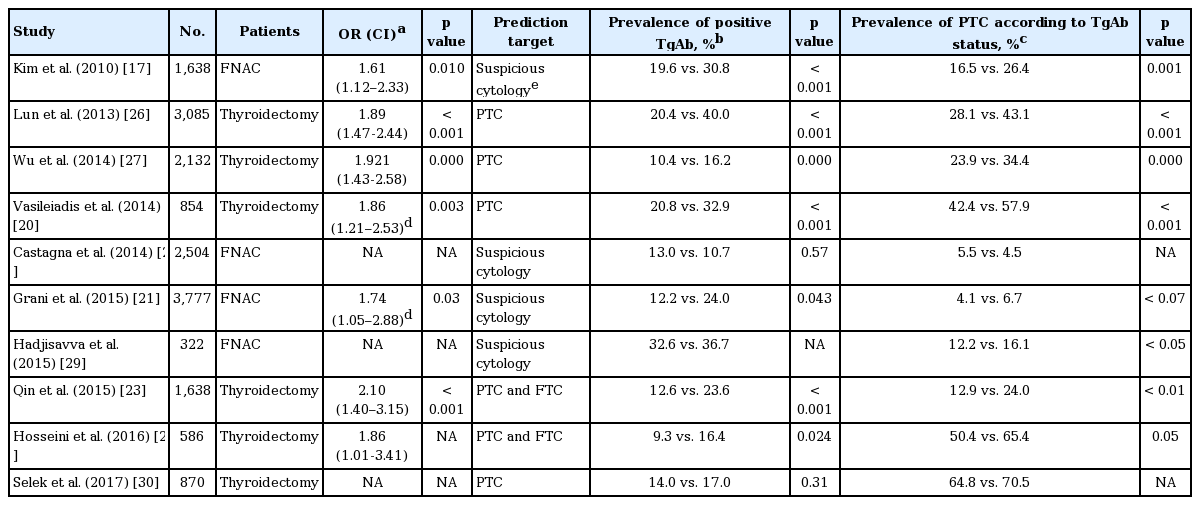
The prevalence of TgAb is approximately 1.5-fold higher in patients with DTC than in the general population with benign nodules (30.8% vs. 19.6%) [17]. TgAb is found more frequently in patients with PTC than in patients with follicular carcinomas [18]. Interestingly, the patterns of TgAb differ between patients with AITD and non-AITD patients, including those with DTC. Latrofa et al. [12] used human recombinant TgAb-Fab to investigate TgAb recognition in sera from patients with non-AITD thyroid diseases (nontoxic multinodular goiter and PTC) and AITD (HT and GD). They found that region A is the major immunodominant region of Tg, because the highest levels of inhibition were induced by TgAb-Fab of region A in all patients (> 50%) regardless of AITD. The inhibition levels were significantly higher in AITD patients than in non-AITD patients [12].
PREOPERATIVE TgAb AS A PREDICTOR OF THYROID CANCER
Positive serum TgAb is more than twice as prevalent in DTC patients than in the general population (25% vs. 10%) [14]. While TPOAb are believed to fix complement and can be used to diagnose HT and hypothyroidism more accurately than TgAb, TgAb may be more tumor-specific than TPOAb [19]. In particular, high TgAb levels can weakly predict thyroid carcinomas in patients with thyroid nodules, unlike TPOAb [17].
One study retrospectively evaluated a total of 2088 patients with thyroid nodules who received fine needle aspiration (FNA), and found that thyroid cancer was significantly associated with positive TgAb (odds ratio [OR], 1.61) [17]. A similar result was obtained by Vasileiadis et al. [20], who found that in patients who received a total thyroidectomy for any reason, preoperative measurements found TgAb more frequently in patients with malignant disease than in those with benign disease (38.2% vs. 19.8%). In another study of 2,562 patients who underwent FNA cytology (FNAC), suspicious cytology was detected more frequently in TgAb-positive patients than in those without TgAb (9.4% vs 5.7%), and isolated TgAb was a mild risk factor for thyroid cancer (OR, 1.74) [21]. Hosseini et al. [22] grouped patients according to TgAb level and found out that the prevalence of malignancy was higher in the positive TgAb group (65.38% vs. 50.42%). Qin el al. [23] also grouped patients according to TgAb level (0, 4, 11, 40, 100, and 500 IU/mL), and found that the prevalence of DTC was significantly higher in the high TgAb groups (≥ 100 IU/mL) than in the low TgAb groups (< 100 IU/mL).
However, when thyroid autoantibodies were considered as a marker for AITD, there was no significant difference in thyroid malignancy rates between AITD and non-AITD groups. Anil et al. [24] recruited patients with newly diagnosed thyroid nodules and evaluated thyroid autoantibodies before FNA. The FNA results revealed no differences in malignancy rates between the non-AITD and AITD groups. However, AITD patients were positive for at least one thyroid autoantibody, so this study could not detect the effects of TgAb alone on malignancy rates. Rago et al. [25] found similar results, but they also did not distinguish between TgAb and TPOAb.
Overall, these results suggest that unlike TPOAb, TgAb could be a weak but confident predictive marker for PTC in patients presenting with thyroid nodules (Table 1) [17,20-23,26-30]. TgAb may detect newly developed epitopes of Tg derived from thyroid cancer cells. Tg from benign follicular cells may be unlikely to develop any novel cancer-specific epitopes. The predictive value of TgAb for thyroid cancer before FNA is not very powerful and is controversial; therefore, other clinical parameters should be combined with TgAb to increase the diagnostic power of TgAb.
PREOPERATIVE TgAb LEVELS IN PREDICTING THE PROGNOSIS OF THYROID CANCER
It is generally accepted that thyroid cancer with AITD has a more favorable prognosis compared to thyroid cancer without AITD [14,16,31]. Most studies, however, have not examined the effects of TPOAb or TgAb separately, but rather the combined effects of both thyroid autoantibodies or the entire AITD pathology. Therefore, studies evaluating the effects of TgAb individually were needed to understand the role of TgAb in cancer prognosis.
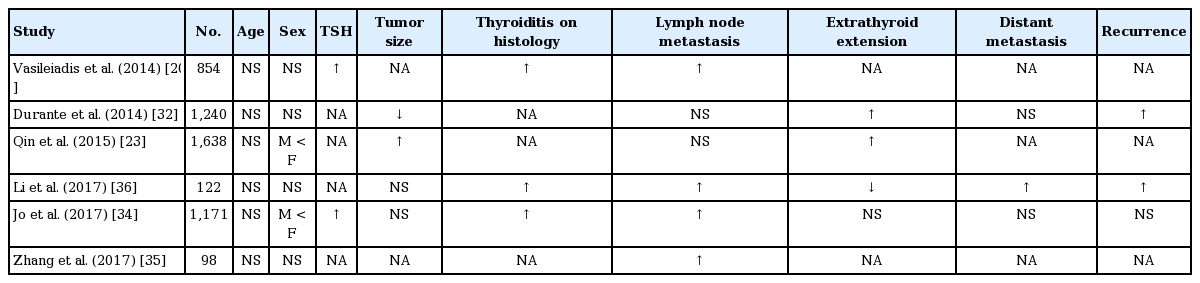
Several studies were conducted on the prognostic effects of TgAb on thyroid cancer in perioperative settings (Table 2). Durante et al. [32] showed that there were more cases of persistent/recurrent disease in thyroid cancer patients with TgAb than in those without TgAb, although these data were not preoperative; instead, TgAb values were obtained 1 to 12 months after thyroidectomy. On the other hand, a study based on a nationwide multicenter registry of thyroid cancer in the United States showed no significant association of TgAb with the stage of disease, disease-free survival, or overall survival [33]. Although these studies found contradictory results, it is interesting to see the potential role of TgAb in the prognosis of thyroid cancer and in its underlying mechanisms.
Associations between preoperative TgAb and initial clinical characteristics and prognosis of thyroid cancer
Since then, there have been several studies reporting a correlation between TgAb and lymph node metastasis in DTC. Vasileiadis et al. [20] reported that DTC patients with TgAb showed more frequent lymph node metastasis than those without TgAb (20.3% vs. 10.0%). Another study also found that elevated preoperative serum TgAb levels in DTC patients were significantly associated with adverse primary tumor characteristics, including nonencapsulated tumors, lymphatic invasion, and lymph node metastasis [34]. Similarly, Zhang et al. [35] found that DTC patients with TgAb had a significantly higher rate of metastasis of cervical lymph nodes, and that lymph node stage was the only independent indicator for persistent TgAb (OR, 3.183). Qin et al. [23] also reported that patients with TgAb showed a higher rate of extrathyroid invasion (30.4% vs. 17.1%, p = 0.031).
One small study by Li et al. [36] evaluated the protein expression of specific invasion-related genes (BRAF V600E and nuclear factor κB [NF-κB]) in thyroid cancer tissues to test whether TgAb is associated with the expression of these genes. The protein expression levels of BRAFV600E and NF-κB were greater in TgAb-positive patients compared to TgAb-negative patients, and a multivariate analysis found that TgAb was an independent factor for predicting lymph node metastasis in thyroid cancer. However, the small number of subjects and the lack of a mechanism for the observed patterns make this result difficult to interpret.
If metastasis or invasion develops from a primary tumor, cancer cells are confined within the site and will induce a cancer-specific immune response, which may in turn cause TgAb levels to rise [37,38]. One recent study used genomic profiling of thyroid cancer to reveal that somatic mutations of Tg derived from metastatic lesions might play a significant role in the pathogenesis of PTC and development of metastasis [39]. Newly developed Tg mutations in this study were associated with a poor prognosis, and we presume these new Tg mutations would induce TgAb. Unfortunately, TgAb was not measured in this study.
POSTOPERATIVE TgAb FOLLOW-UP
High TgAb levels could cause Tg levels to be undetectable. Because of this, follow-up measurements of TgAb levels are useful for detecting persistent or recurrent disease after total thyroidectomy and RI remnant ablation of DTC [4]. Therefore, rising TgAb levels after initial treatment are evaluated as a ‘biochemical incomplete response,’ and these patients should be carefully monitored to detect recurrent or persistent disease [4].
TgAb levels may transiently rise postoperatively as an apparent immune reaction to released tissue particles after surgery, and they may rise after RI [40]. After total thyroidectomy and RI, all antibodies disappeared progressively, with a median disappearance time for TgAb of about 3 years [40,41]. Although the pathophysiologic importance of TgAb after total thyroidectomy is unclear, the persistence of TgAb or increasing titers more than 1 year after thyroidectomy and RI probably indicates the presence of residual thyroid tissue, and possibly an increased risk of recurrence [14]. Furthermore, sequential changes in TgAb titers are a reliable predictor of disease prognosis and therefore serve as a useful factor for clinical decision-making [42]. In a study of thyroid cancer patients with undetectable serum Tg concentrations, 18% of patients with serum TgAb concentrations > 100 U/mL had a recurrence, compared with only 1% of patients with serum TgAb ≤ 100 U/mL [43].
We previously reported that in DTC patients who previously had total thyroidectomy and high-dose RI remnant ablation, thyroid bed RI uptake at the postoperative 6- to 12-month whole-body scan showed a close association with higher TgAb levels, compared to no thyroid bed uptake [44]. Dewi et al. [45] also reported that higher post-RI TgAb levels were significantly correlated with unsuccessful RI therapy (OR, 5.379; p = 0.007), which indicates that TgAb could serve as a surrogate marker for successful RI. We assume that the existence of TgAb represents remnant thyroid tissue, because TgAb can be detected as a response to remaining Tg.
A study by Trimboli et al. [46] evaluated the prognostic impact of pre-RI TgAb levels on long-term outcomes for TgAb-positive DTC patients. Positive TgAb levels before RI increased the risk of disease recurrence over time (OR, 3.57), and disease-free survival was higher in TgAb-negative patients (hazard ratio, 2.59).
FNA WASHOUT TG AND SERUM TgAb LEVELS
FNAC of suspicious lymph nodes in DTC patients frequently shows false-negative results due to easily missed cancer cells in cases with an insufficient number of metastatic cancer cells within the lymph nodes. Therefore, measuring Tg levels in FNA washout (FNA-Tg), in addition to FNAC, has been the best supplementary diagnostic tool to increase the diagnostic accuracy of FNAC [47]. Increased serum TgAb levels could lower serum Tg concentrations, so measurements of Tg should always be accompanied by TgAb testing, as indicated by clinical management guidelines [4]. This leads to the question of whether increased serum TgAb levels could interfere with FNA-Tg levels in metastatic lymph nodes of DTC patients.
Several studies found that the presence of serum TgAb could lower FNA-Tg levels, and therefore measuring serum TgAb levels may be helpful in DTC patients who are bound to FNAC with FNA-Tg on suspicious lymph nodes [48-51]. One study demonstrated that when applying the same FNA-Tg cutoff used in the TgAb-negative group to the TgAb-positive group to detect metastatic lymph nodes in DTC patients, the sensitivity and positive predictive value of FNA-Tg were reduced by more than 10% [51]. A similar result was obtained in a postoperative setting: the TgAb-positive group showed significantly lower FNA-Tg levels than the TgAb-negative group in metastatic lymph nodes, and thus showed lower sensitivity and negative predictive values with the same FNA-Tg levels [49].
In multiple studies, however, high serum TgAb levels showed no interference with FNA-Tg sensitivity [52,53]. Duval et al. [54] reported that the measurement of FNA-Tg is useful for evaluating suspicious lymph nodes in patients with DTC, regardless of TgAb. Boi et al. [53] measured TgAb levels in FNA washout fluid in a limited number of patients, and found that TgAb was undetectable in patients with negative serum TgAb, but a small number of patients with positive serum TgAb (two of eight) showed positive TgAb in washout fluid. FNA-Tg levels from metastatic lymph nodes were lower than those found in TgAb-negative FNA washout fluids, but this difference might not be significant in the diagnostic process. One explanation for why FNA-Tg levels are much higher than TgAb levels in FNA washout fluid is that it may overcome interference by TgAb, due to high Tg levels in metastatic lymph nodes leading to saturated TgAb-binding sites. Thus, the measurement of serum TgAb at the time of FNA-Tg is not recommended in current clinical management guidelines [4]. Additional studies are needed to confirm the interfering effects of serum TgAb levels on FNA washout Tg levels.
CONCLUSIONS
It remains challenging to interpret the diagnostic and prognostic implications of TgAb levels before surgery for DTC patients with TgAb, although basic and clinical researchers have uncovered secret messages from this antibody over the last two decades. Future research should focus on understanding the epitope-specific immunological responses of TgAb in thyroid cancer, as several studies have suggested unique immunological roles for TgAb.
Notes
No potential conflict of interest relevant to this article was reported.