Hepatitis C virus infection in chronic kidney disease: paradigm shift in management
Article information
Abstract
Hepatitis C virus (HCV) infection in chronic kidney disease (CKD) is associated with increased liver-related morbidity and mortality rates, accelerated progression to end-stage renal disease, and risk of cardiovascular events. CKD patients with HCV infection require antiviral therapy. Pegylated interferon (peg-IFN) plus ribavirin was the standard of care for HCV-infected CKD patients before the introduction of first-generation direct-acting antiviral (DAA) oral anti-HCV agents. Peg-IFN-based treatment has a low virologic response rate and poor compliance, resulting in a high dropout rate. Recently, several clinical trials of all-DAA combination regimens have reported excellent antiviral efficacy and few adverse drug reactions in HCV-infected patients with CKD. These positive results have revolutionized the treatment of chronic HCV infection in this population. In this review, we address the impact of chronic HCV infection in CKD patients, and discuss their management using next-generation DAAs.
INTRODUCTION
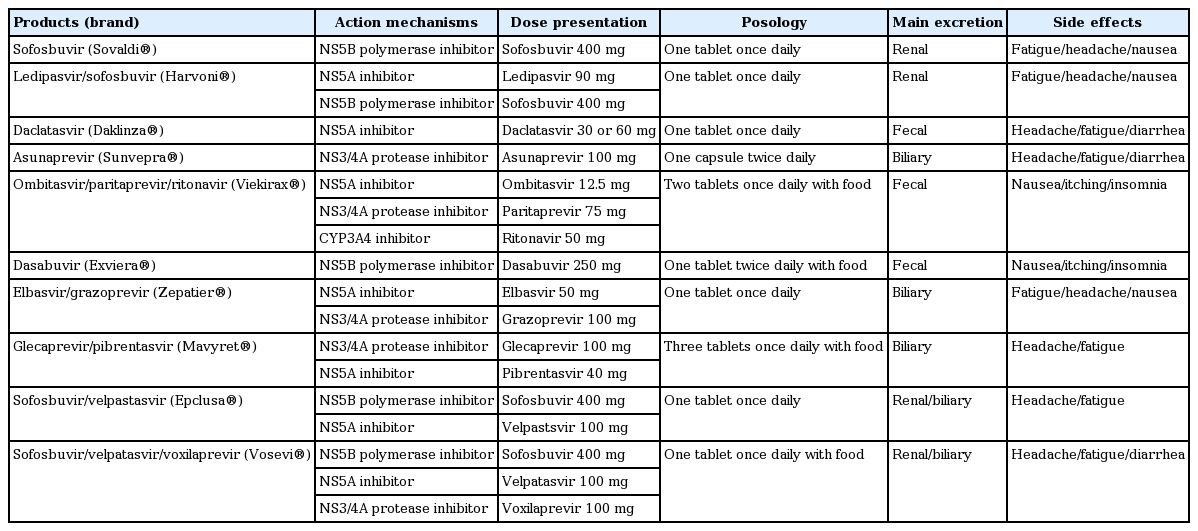
Hepatitis C virus (HCV) infection is associated with high rates of liver-related morbidities and mortality. Public interest in HCV is growing, as more than 180 million people, 2.8% of the global population, are infected with HCV [1,2]. The hepatic complications of HCV infection including liver cirrhosis, hepatic decompensation, and hepatocellular carcinoma are well documented. However, 40% to 70% of cases of HCV infection are accompanied by extrahepatic manifestations such as autoimmune, metabolic, renal, cardiovascular, central nervous system, and lymphoproliferative disorders [3-5]. Kidney disease in particular is a common extrahepatic manifestation of HCV infection. Chronic HCV infection is related not only to chronic kidney disease (CKD) but also accelerates renal deterioration, leading to end-stage renal disease (ESRD) [6-8]. In addition, HCV infection increases the morbidity and mortality rates of both dialysis patients and kidney transplant (KT) recipients [9-11]. Conventional interferon (INF)-based therapy for HCV infection has a relatively low virologic response rate and poor tolerability in CKD patients [12,13]. The recent development of novel direct-acting antiviral (DAA) agents has revolutionized the treatment of HCV infection (Table 1), especially for CKD patients, a difficult to treat population.
In this review, we address the clinical impact of HCV infection in patients with CKD including predialysis patients, those on dialysis, and KT recipients. We also provide a brief overview of conventional therapies and discuss novel antiviral therapies for HCV infection.
CLINICAL IMPACT OF HCV INFECTION IN CKD PATIENTS
HCV in patients with CKD
Although patients with CKD, who are immunocompromised, are expected to have a high prevalence of HCV infection, few large-scale epidemiologic studies in diverse geographical locations involving predialysis patients with CKD have been performed. A recent study in Taiwan by Lee et al. [14] reported a 7.6% prevalence of HCV infection in 4,185 predialysis patients with CKD. In this study, the prevalence of HCV increased with CKD stage, and the 5-year cumulative incidence of ESRD was 52.6% and 38.4% in patients with CKD with and without HCV infection, respectively. However, another Taiwanese study reported that the prevalence of CKD is higher in patients with than without HCV infection (5.46% vs. 3.43%, respectively), with an adjusted hazard ratio (aHR) of 1.28 (95% confidence interval [CI], 1.12 to 1.46) [15]. These results suggest a correlation between HCV and renal function, indicating that HCV infection can lead to CKD and promote progression from CKD to ESRD. Various hepatic and/or extrahepatic manifestations of HCV infection can contribute to renal deterioration. Insulin resistance is associated with HCV-induced hepatic dysfunction and has been found in 10% to 60% of HCV-infected patients [16-18]. However, recent experimental studies have demonstrated that the HCV core protein interferes with insulin signaling by degrading insulin receptor substrate-1 by upregulating the expression of mammalian target of rapamycin (mTOR)/p70 ribosomal protein S6 kinase 1, suppressor of cytokine signaling 3, and tumor necrosis factor-α [19,20]; and downregulating glucose transporter 2,4 expression through upregulation of glucose-6-phosphatase and phosphoenolpyruvate carboxykinase 2 [21,22]. Sciacqua et al. [23] reported higher fasting glucose, homeostasis model assessment of insulin resistance (HOMA-IR), and triglyceride levels in HCV-infected patients and a significant correlation between the HOMA-IR and estimated glomerular filtration rate (eGFR). Insulin resistance is also correlated with type 2 diabetes mellitus and hyperlipidemia, leading to atherosclerosis and cardiovascular events in HCV-infected patients. This implies that insulin resistance and its vascular complications directly and/or indirectly promote renal deterioration. Chronic HCV infection induces an immune response and a direct cytopathic effect. HCV can enter and replicate in hepatocytes and B-lymphocytes. HCV envelope 2 protein can bind to the CD81 transmembrane 4 family glycoprotein on B-lymphocytes, resulting in their activation [24]. Moreover, the HCV nonstructural (NS) region 5A and core protein, which mimic auto-antigens, can stimulate B-lymphocytes. Persistently stimulated B-lymphocytes produce immunoglobulin G (IgG)-HCV complexes, resulting in the generation of rheumatoid factor-IgM and cryoglobulins [24,25]. Such immune complexes are implicated in the pathogenesis of glomerulonephritis (GN) in HCV-infected patients [24-26]. Although the most common HCV-related GN is type 1 membranoproliferative glomerulonephritis (MPGN) with/without cryoglobulin, other types of GN such as membranous nephropathy (MGN), focal segmental glomerulosclerosis, IgA nephropathy, fibrillary GN, thrombotic microangiopathy, and tubulointerstitial nephropathy have been described [26-28]. In an Italian multicenter study, 87% of patients with cryoglobulinemia had HCV infection. In addition, 83% of these patients showed the MPGN pattern on kidney biopsy [29]. El-Serag et al. [30] reported that the prevalence of cryoglobulinemia (0.57% vs. 0.05%) and MPGN (0.36% vs. 0.05%) was higher in patients with than without HCV using Veterans Administration data. However, HCV infection presents as only microalbuminuria in > 10% of HCV-infected patients [31], suggesting that HCV has a greater influence on renal deterioration through GN than formerly believed. Therefore, patients infected with HCV should undergo regular surveillance for the early detection of GN.
HCV in patients on dialysis
According to the 2004 Dialysis Outcomes and Practice Patterns Study (DOPPS), the prevalence of HCV infection in patients on hemodialysis (HD) is 13.5%. This study included 308 dialysis facilities in America, Europe, and Asia, and found differences among regions of 2.6% to 22.9% [32]. In 2009, Johnson et al. [33] investigated the prevalence of HCV in dialysis patients in 10 Asia-Pacific countries and reported an incidence of 7.9% in HD patients and 2.0% in peritoneal dialysis patients. In addition, the dialysis modality was associated with the prevalence of HCV infection. After publication of the Recommendations for Preventing Transmission of Infections among Chronic Hemodialysis Patients by the Centers for Disease Control in 2001, the prevalence of HD-related viral transmission has decreased [34]. HD patients typically acquire HCV during HD, direct contact between patients, a breach in infection control, contaminated equipment, or transfusion of contaminated blood products [35,36]. The DOPPS study showed that the prevalence of HCV increases with the duration of HD (58% in > 20 years), and that ethnicity (black), hepatitis B co-infection and substance/alcohol abuse are risk factors for HCV infection [32]. These results suggest a lack of awareness of infection control in dialysis units and emphasize the importance of continuous surveillance for HCV. HD patients with HCV infection have higher rates of morbidity and mortality than those without HCV [37-39]. Goodkin et al. [39] analyzed the hospitalization rate, mortality rate, and quality of life of 76,689 HD patients with HCV using the DOPPS data. The incidence of hospitalization was significantly higher in HD patients with HCV compared to those without HCV. Moreover, the aHR in HD patients with HCV versus those without HCV for all-cause, hepatic-related, cardiovascular-related, and infection-related hospitalization was 1.09 (95% CI, 1.04 to 1.13), 4.40 (95% CI, 1.03 to 1.17), 1.10 (95% CI, 1.03 to 1.17), and 1.08 (95% CI, 1.00 to 1.18), respectively. Similarly, the mortality rate was higher in HD patients with HCV, and the aHRs for HD patients with versus those without HCV for all-cause and hepatic-related mortality were 1.12 (95% Cl, 1.05 to 1.20) and 5.90 (95% CI, 3.67 to 9.50), respectively. The mortality rate due to cardiovascular or infectious disease was non-significantly higher in HD patients with HCV. The Kidney Disease Quality of Life (KDQOL)-36 quality of life scores were also lower in HD patients with HCV across all physical and mental domains.
HCV in KT recipients
The prevalence of HCV infection in KT recipients varies geographically from 6% to 46%. Notably, KT recipients typically become infected with HCV by dialysis rather than during or after transplantation [40]. To date, most studies have shown that HCV infection reduces graft and patient survival after KT [10,11,40,41]. New-onset diabetes mellitus after transplant (NODAT) frequently occurs in KT recipients. Immunosuppressants such as steroids, calcineurin inhibitors (tacrolimus and cyclosporin), and mTOR inhibitors (sirolimus and everolimus) also contribute to the development of NODAT in KT recipients [42-45]. Kasiske et al. [41] reported a higher prevalence of NODAT in KT recipients with than without HCV infection (25.6% vs. 15.4%, respectively). A meta-analysis of 2,502 KT recipients showed an adjusted odds ratio for NODAT of 3.97 (95% CI, 1.83 to 8.61) [17]. Similar to predialysis CKD, these data suggest that HCV infection-related NODAT increases cardiovascular and infection risk, which reduces graft and patient survival after KT [41-45]. Allograft GN accounts for approximately 18% to 22% of graft loss [46-48]. HCV infection is strongly associated with development of allograft GN after KT, and the MPGN and MGN. Hammoud et al. [46] showed a higher prevalence of MPGN in KT recipients with HCV compared to those without HCV (5.9% vs. 2.8%, respectively). Cruzado et al. [47] reported a 45.8% incidence of MPGN using 44 biopsies of KT recipients with HCV and suspected allograft GN. HCV infection reduces graft survival in KT recipients [10,11,17,43,48]. A meta-analysis of 18 observational studies involving 133,530 KT recipients showed that HCV was a significant independent risk factor for graft loss with a relative risk of 1.76 (95% CI, 1.46 to 2.11) [10]. Scott et al. [11] reported a high incidence of graft failure in 140 KT recipients. The aHR for graft loss was 1.71 (95% CI, 1.28 to 2.29) for KT recipients with HCV; GN, chronic allograft nephropathy, and death were the most frequent causes of graft failure. Similarly, the majority of studies have reported that KT recipients with HCV have a low survival rate. Scott et al. [11] also reported a 1.8% (n = 140) incidence of HCV infection in 7,572 KT recipients. The survival rate of KT recipients with and without HCV was 77% versus 90% and 50% versus 79% at 5 and 10 years, respectively. The aHR for mortality was 2.38 (95% CI, 1.69 to 3.37). The rates of mortality due to cardiovascular disease (aHR, 2.74), malignancy (aHR, 2.52), and hepatic failure (aHR, 2.21) were high. Therefore, not only hepatic complications but also cardiovascular disease and cancer contribute to the death of KT recipients with HCV. Early aggressive treatment of HCV infection can reduce mortality by ameliorating extrahepatic complications such as cardiovascular diseases, cancer, and renal diseases in addition to hepatic complications.
ANTIVIRAL THERAPIES IN CKD PATIENTS WITH HCV INFECTION
CKD patients with chronic HCV infection require antiviral therapy; however, few treatment options were available until recently. Pegylated interferon (peg-IFN) plus ribavirin was the standard of care for HCV-infected patients before introduction of the first-generation DAAs, telaprevir and boceprevir (HCV-NS3/NS4A protease inhibitors). Several clinical trials of peg-IFN plus low-dose ribavirin have involved renal transplant candidates undergoing maintenance HD. Rendina et al. [49] reported a high sustained virologic response (SVR) rate and low dropout rate in a clinical trial of peg-IFN alfa-2a plus low-dose ribavirin in hemodialyzed patients infected with HCV awaiting renal transplant. Another randomized controlled trial showed that peg-IFN plus ribavirin combination therapy has greater efficacy and safety than peg-IFN monotherapy for HCV in patients on HD [50,51]. Fabrizi et al. [52] conducted a systematic review and meta-analysis of 11 clinical studies on the efficacy and safety of IFN-based combination therapy for HCV-infected patients on regular dialysis. The summary estimate SVR and dropout rate was 0.60 and 0.18, respectively, and anemia and infection were the major barriers to therapy continuation. Triple antiviral therapies comprising peg-IFN, low-dose ribavirin and telaprevir or boceprevir have been tested in HCV-infected patients with CKD [53-55]. The interpretation of the data from these trials was hampered by the small numbers of enrolled patients and involvement of only those infected with HCV genotype 1. In addition, side effects unrelated to peg-IFN and ribavirin including drug-drug interactions, cutaneous rash, anemia, and poor compliance hampered therapy continuation. The goal of anti-HCV treatment is an undetectable viral load at least 12 weeks after completing therapy (SVR12), which is a surrogate marker for virologic cure. IFNbased regimens are not sufficient in terms of SVR and safety in difficult to treat patients such as those with renal impairment. Several clinical trials using next-generation DAAs have been performed. Roth et al. [56] performed a phase III, prospective, randomized trial (the C-SURFER study) of the safety and efficacy of elbasvir (HCV-NS5A inhibitor) plus grazoprevir (HCV-NS3/NS4A protease inhibitor) in treatment-naïve and -experienced patients with HCV genotype 1 infection and stage 4 to 5 CKD; the SVR12 rate was 99% and the incidence of adverse events was low. Saxena et al. [57] evaluated the safety and efficacy of sofosbuvir (HCV-NS5B polymerase inhibitor)-containing regimens (PEG-interferon, simeprevir [HCV-NS3/NS4A protease inhibitor], and ribavirin) in HCV-infected patients with impaired renal dysfunction. This real-world outcome study (the HCV-TARGET study) showed SVR12 rates of 81% to 88% depending on the GFR; however, patients with a GFR of ≤ 45 mL/min/1.73 m2 more frequently experienced worsening renal function and ribavirin-related hemolytic anemia. Based on the renal excretion of sofosbuvir and its metabolites, the use of this DAA agent is not recommended in patients with a GFR of ≤ 30 mL/min/1.73 m2. Pockros et al. [58] performed a clinical trial (the RUBY study) of the combination of ombitasvir (HCV-NS5A inhibitor), paritaprevir (HCV-NS3/NS4A protease inhibitor), ritonavir (HCV-NS5A inhibitor), and dasabuvir (HCV-NS5B polymerase inhibitor) in 20 genotype 1 HCV-infected patients with stage 4 to 5 CKD or ESRD; the SVR12 rate was 90% and all adverse events were tolerable. If ribavirin is added to maximize therapeutic efficacy in patients infected with genotype 1a HCV, dose reduction or interruption of ribavirin may be required to control progressive anemia that requires erythropoietin supplementation. This treatment regimen is in clinical use for genotype 1 HCV-infected patients with severe renal dysfunction. Gane et al. [59] reported good results from the EXPEDITION-4 study of the clinical efficacy of glecaprevir (HCV-NS3/NS4A protease inhibitor) and pibrentasvir (HCV-NS5A inhibitor) in 104 HCV-infected patients with stage 4 to 5 CKD or ESRD. This combined regimen showed an SVR12 rate of 98% with minimal adverse events. Moreover, treatment-naïve and -experienced patients infected with HCV of any genotype achieved excellent antiviral outcomes. As glecaprevir is an inhibitor of the viral protease NS protein 3/4A and pibrentasvir is an inhibitor of the NS protein 5A of HCV, this DAA combination regimen has pan-genotypic coverage. Although this was not a blinded and randomized trial and enrolled only one patient infected with HCV genotype 5/6, this is the only pan-genotypic regimen approved for the treatment of HCV-infected patients with kidney disease. The MAGELLAN-2 study conducted by Reau et al. [60] was a phase 3, single-arm, open-label, multicenter trial of the efficacy and safety of glecaprevir/pibrentasvir for genotype 1 to 6 HCV-infected non-cirrhotic patients who had undergone primary liver or kidney transplantation. The SVR rate was 98% and adverse events were tolerable without DAA dosage modification or discontinuation. In addition, no potential drug interactions of glecaprevir/pibretasvir with concomitant immunosuppressive agents were identified. In 2017, The American Association for the Study of Liver Disease and the Infectious Diseases Society of America jointly updated the HCV treatment guidelines to recommend combination therapy with glecaprevir/pibrentasvir for 12 weeks for the treatment of chronic HCV genotype 1 to 6 infections in liver transplant patients without cirrhosis. Fig. 1 shows a treatment algorithm for CKD patients using DAAs according to HCV genotype and eGFR.
CONCLUSIONS
The recent advent of next-generation DAA agents has revolutionized the treatment of patients with chronic HCV infection in terms of the viral eradication rate and the tolerability of adverse reactions. Indeed, in HCV-infected patients with advanced renal impairment, the clinical efficacy and safety of DAA combination regimens are excellent. Furthermore, the development of a pan-genotypic DAA combination regimen, glecaprevir and pibrentasvir, has increased the efficacy of treatment. The availability of DAA agents for HCV-infected patients with CKD, ESRD, or on HD enables selection of patients and the optimal time to start treatment. A prospective study is needed to evaluate the long-term outcomes (such as progression to advanced-stage CKD, dialysis-phase ESRD, and graft and KT recipient survival) of all-DAA combination regimens in HCV-infected CKD patients.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This study was financially supported by the research fund of Dankook University in 2015.