Negative pathology after endoscopic resection of gastric epithelial neoplasms: importance of pit dysplasia
Article information
Abstract
Background/Aims
Endoscopic resection (ER) is a well-established treatment modality for gastric epithelial neoplasm. However, there is a discrepancy between forceps biopsy and ER specimen pathology, including a negative pathologic diagnosis (NPD) after ER. It has been suggested that pit dysplasia (PD) is a subtype of gastric dysplasia, and the aim of this study was to assess the significance of PD in cases with NPD after ER for early gastric neoplasms.
Methods
After ER, 29 NPD lesions that had an associated pretreatment forceps biopsy specimen, were correctly targeted during ER, and had no cautery artifact on the resected specimen were included in this study.
Results
Sixteen lesions showed PD and 13 had no neoplastic pathology. The initial pretreatment forceps biopsy diagnoses of 29 NPD lesions were low-grade dysplasia (LGD) in 17 lesions, high-grade dysplasia (HGD) in seven lesions, and adenocarcinoma in five lesions, which after review were revised to PD in 19 lesions, LGD in four lesions, adenocarcinoma in two lesions, and no neoplastic pathology in four lesions. Overall, nine lesions (31%) were small enough to be removed by forceps biopsy, four NPD lesions (14%) were initially misinterpreted as neoplastic lesions, and 16 PD lesions (55%) were misinterpreted as NPD lesions on ER slides.
Conclusions
Approximately half of the lesions initially diagnosed as LGD or HGD were subsequently classified as PD. Therefore, including PD as a subtype of gastric dysplasia could reduce the diagnostic discrepancy between initial forceps biopsy and ER specimens.
INTRODUCTION
Endoscopic resection (ER), including endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD), is widely accepted as a first-line modality for gastric epithelial neoplasms, including gastric epithelial dysplasia (GED) and early gastric carcinoma (EGC) [1-4]; ER is also considered to be an improved diagnostic procedure for early gastric neoplasms [5]. In view of histopathological diagnosis, issues regarding a discrepancy between forceps biopsy and ER specimens, including a negative pathologic diagnosis (NPD) after ER, have been rising [6-8]. When a discrepancy occurs, there can be either a higher or a lower grade after ER compared to initial forceps biopsy. Takao et al. [6] reported that 49.0% of cases classified as borderline (adenoma or regenerative atypia) in a forceps biopsy specimen were subsequently diagnosed as adenocarcinoma in the ER specimen. Furthermore, Lim et al. [7] reported an overall of discrepancy rate of 31.7%, and 23.9% of these cases showed a higher grade after ER, and Kim et al. [8] reported that 3.2% of cases showed NPD after ER.
In cases where a NPD is made after ER, there are three possible explanations: (1) the tumor was been completely removed via forceps biopsy because of its small size; (2) there were discordant histopathological findings between forceps biopsy and ER specimens; and (3) there was a sampling error or mistargeting during ER. A number of histopathological factors may be involved when discrepancies occur, including the type of GED. GED is defined according to its architectural and cytological abnormalities along the full length of the pit and the surface epithelium, and is categorized into adenomatous (or intestinal), foveolar (or gastric), and hybrid types based on its morphological characteristics [9-11]. However, several recent studies reported pit or crypt dysplasia, which exhibited dysplasia or atypical glandular or crypt lesions with surface maturation in Barrett esophagus [12,13] and the stomach [14-16]. With respect to the discrepancy that can occur between forceps biopsy and ER specimens, pit dysplasia (PD) might be diagnosed as GED in forceps biopsy and as a negative pathology after ER, and this could explain why NPD is sometimes found in ER specimens. In light of this, we reviewed the clinicopathological features of cases where there was an NPD after ER for gastric neoplasms, and evaluated the significance of PD in these cases.
METHODS
Patients
A cohort of 3,381 gastric epithelial neoplasms (including GEDs and EGCs) from 2,972 patients who underwent EMR or ESD at Pusan National University Hospital (Busan, Korea) between 2006 and 2013 were included in this study. We defined NPD as no specific neoplastic gastric lesion after ER in cases of neoplastic lesions diagnosed on pretreatment forceps biopsy, such as GED or adenocarcinoma. After reviewing the clinical information and pathologic reports of these patients, a total of 86 lesions (2.5%) that had no specific gastric neoplastic findings after ER were further evaluated. Of these, a comparison of histopathological features between forceps biopsy and ER specimens was possible in 33 lesions, but not for the other 53 lesions because the forceps biopsy had been performed in other hospitals. Of the 33 lesions with available pretreatment forceps biopsy and ER slides, four further cases were excluded due to mistargeting (n = 3) during ER, or a marked thermal injury on the ER specimen (n = 1). Finally, 29 NPD lesions in 29 patients after ER with matched forceps biopsy and ER slides available for review were included in this study (Fig. 1). The study protocol was reviewed and approved by the Institutional Review Board at Pusan National University Hospital (E-2014167).
Endoscopic procedures
The locations of each lesion was classified based on its longitudinal direction (upper, middle, or lower third region of the stomach). The macroscopic shapes of lesions were categorized as either protruding (I), non-protruding and non-excavated (II), or excavated (III). Type II lesions were subclassified as slightly elevated (IIa), flat (IIb), or slightly depressed (IIc). All lesions were also classified into three groups: elevated (I, IIa), flat (IIb), and depressed (IIc, III) types. Lesion color was categorized as similar, discolored, or reddish compared to the surrounding non-neoplastic mucosa.
ER procedures were performed by two experienced endoscopists (G.H.K. and G.A.S.), using a single-channel endoscope (GIF-H260 or GIF-Q260, Olympus Co. Ltd., Tokyo, Japan). Procedures were performed with the patient under conscious sedation with cardiorespiratory monitoring. For sedation, midazolam (5 to 10 mg) and meperidine (25 mg) were administered intravenously. Propofol was administered as needed during the procedure. Either EMR or ESD was performed as the ER procedure. For EMR, argon plasma coagulation was used to mark the borders of the lesion, which had been identified via conventional endoscopy or chromoendoscopy with the application of an indigo carmine solution. After marking, a saline solution (0.9% saline with a small amount of epinephrine and indigo carmine) was injected submucosally around the lesion in order to elevate it off the muscular layer. The lesion was then resected using a snare. For ESD, a circumferential mucosal incision was made outside the marking dots with an IT knife (Olympus) and/or a Flex knife (Olympus) after marking the borders of the lesion by argon plasma coagulation. Next, submucosal dissection was performed, using the knife to completely resect the lesion. If necessary during the procedure, the submucosal injection was repeated and endoscopic hemostasis was achieved. A high-frequency electrosurgical current generator (Erbotom VIO 300D, ERBE, Tübingen, Germany) was used during marking, mucosal incision, submucosal dissection, and hemostasis.
All patients were recommended to have a follow-up endoscopy in the subsequent 3 to 6 months and annually thereafter. On follow-up endoscopy, a detail examination was performed not only to detect recurrence at the previous resection site but also to screen for other lesions of the stomach.
Histopathological evaluation
Resected specimens were fixed in formalin and serially sectioned at 2-mm intervals in order to assess tumor involvement in the lateral and vertical margins. The tumor size was macroscopically measured in the resected specimens. The histopathological classification and grade of the lesion were evaluated based on the Vienna classification for gastrointestinal (GI) neoplasia [17]. PD was defined by the presence of mild or marked cytological atypia in the gastric pits, including nuclear pleomorphism and stratification, increased nuclear/cytoplasmic ratio, nuclear size and irregularity, hyperchromasia, and increased mitosis without involvement of the surface epithelium, as previously described [14-16]. Based on architectural complexity and cytological atypia, each lesion was graded as either low or high according to previously established criteria [18].
Pretreatment forceps biopsy and ER slides were reviewed independently by two expert GI pathologists (A.K. and D.Y.P.), and if their diagnoses did not coincide, a consensus diagnosis was made using a multiheaded microscope.
RESULTS
Clinicopathological characteristics of patients and lesion
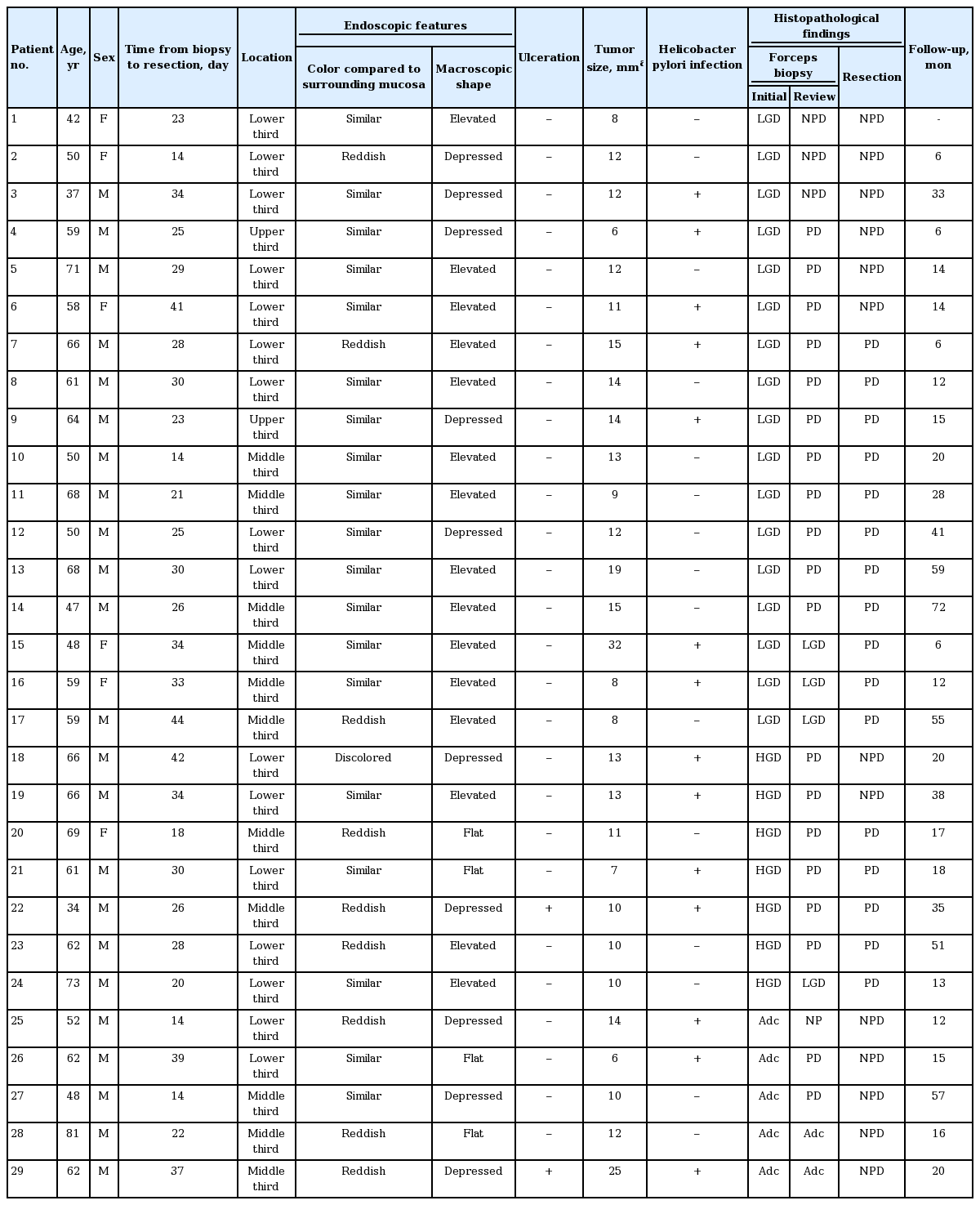
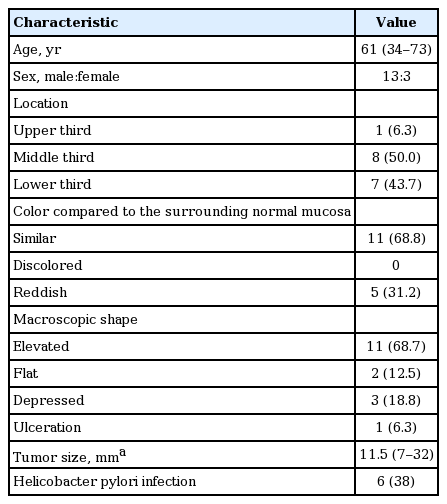
The clinicopathological characteristics of the patients and lesions are summarized in Table 1. The patients included 23 men and six women, with a median age of 61 years (range, 34 to 81). Of 29 NPD lesions, 16 lesions were located in the lower third, 11 were in the middle third, and two were in the upper third region of the stomach. Compared to the surrounding normal mucosa, the color of lesions was similar in 19 lesions, discolored in one lesion, and reddish in nine lesions. Macroscopically, 15 lesions were classified as elevated, four as flat, and 10 as depressed. Ulceration was found in two lesions, and Helicobacter pylori infection was present in 14 lesions. The median tumor size, macroscopically measured in the resected specimens, was 12 mm (range, 6 to 32). The median duration between forceps biopsy and subsequent ER was 28 days (range, 14 to 44).
Histopathological review
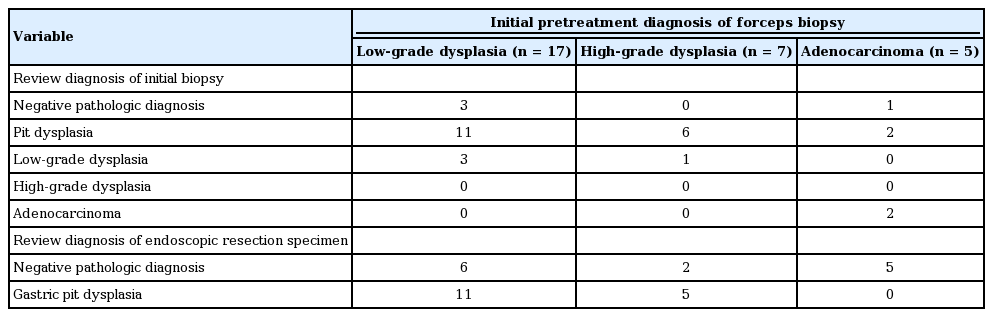
The initial pretreatment forceps biopsy diagnoses of 29 NPD lesions were low-grade dysplasia (LGD) in 17 lesions (58.6%), high-grade dysplasia (HGD) in seven lesions (24.1%), and adenocarcinoma in five lesions (17.3%). After a review of the initial pretreatment forceps biopsy by expert GI pathologists, these diagnoses were PD in 19 lesions (65.5%), LGD in four lesions (13.8%), adenocarcinoma in two lesions (6.9%), and no neoplastic pathology (chronic gastritis with regenerative atypia) in four lesions (13.8%) (Table 2). ER slides of 29 NPD lesions were further evaluated by expert GI pathologists; of these, 16 lesions showed cytological atypia in the gastric pits without surface epithelium involvement. Therefore, the final diagnoses were PD in 16 lesions (55.2%) and no neoplastic pathology in 13 lesions (44.8%).
Comparison of the diagnoses of initial/reviewed forceps biopsy and reviewed endoscopic resection specimen.
Fig. 2 summarizes the relationship between the initial and reviewed diagnoses of the pretreatment forceps biopsy specimens, and the reviewed diagnoses of ER specimens. Of 16 lesions that were finally diagnosed as PD in the ER specimen, 11 lesions were diagnosed as LGD and five as HGD based on the initial biopsy specimen, although the initial biopsy diagnosis was further changed to PD after review. These diagnostic differences might be due to the tangential sectioning of the biopsy specimen, making it difficult to identify the exact orientation of the gastric mucosa and surface maturation of the gastric dysplastic lesions (Fig. 3). Of 13 lesions that finally had NPD on ER slides, six lesions were diagnosed as LGD, two as HGD, and five as adenocarcinoma based on the initial biopsy specimen. Therefore, nine lesions (seven PD lesions and two adenocarcinoma lesions) were completely removed via forceps biopsy because of their small size.

The relationship between the initial and reviewed diagnoses of the pretreatment forcep biopsy specimens, and the reviewed diagnoses of endoscopic resection specimens in 29 cases with negative pathology after endoscopic resection. LGD, low-grade dysplasia; NPD, negative pathologic diagnosis; PD, pit dysplasia; HGD, high-grade dysplasia; Adc, adenocarcinoma.

Endoscopic and histologic findings of gastric pit dysplasia. (A) A slightly elevated lesion with similar color to the surrounding mucosa is seen at the gastric angle (arrow). (B) Histologic examination by endoscopic forceps biopsy shows that the dysplasia is composed of tubules lined by columnar cells with hyperchromatic, pencillate nuclei exhibiting pseudostratification (H&E, ×40). It was therefore diagnosed as low-grade dysplasia. (C) However, it is difficult to establish the orientation of the gastric pits (H&E, ×400). (D) The specimen resected by endoscopic submucosal dissection. (E, F) Histologic examination of the resected specimen reveals gastric dysplastic changes in the lower portion of the pits that is continuous with surface foveolar maturation (E: H&E, ×40; F: H&E, ×400).
Overall, our data showed that of 29 lesions with NPD after ER, nine pathologic lesions (31.0%) were removed via forceps biopsy, four NPD lesions (13.8%) were initially misinterpreted as neoplastic lesions, and 16 PD lesions (55.2%) were misinterpreted as NPD lesions in the ER specimen.
Clinicopathological characteristics of lesions finally diagnosed as pit dysplasia
Of 16 lesions finally diagnosed as PD after ER, almost all were located in the middle or lower third region of the stomach. Approximately two-thirds of the PD lesions had similar color to that of the surrounding normal mucosa, and had an elevated macroscopic shape (Table 3). Ulceration was found in one lesion, and H. pylori infection was present in six lesions (38%). The median tumor size was 11.5 mm (range, 7 to 32).
Follow-up
Follow-up endoscopy over at least 6 months was performed in 28 patients. In two patients with adenocarcinoma, after pretreatment biopsy had been reviewed, chest radiography, abdominal computed tomography, and laboratory measurements of tumor markers were performed 6 months after ESD and annually thereafter. During the median follow-up period of 17.5 months (range, 6 to 72), there was no recurrence at the previous resection site, but a metachronous early gastric cancer occurred in one patient with PD (50 months after ESD), which was treated by ESD.
DISCUSSION
In the present study, we reviewed the 29 lesions that had NPD after ER in spite of neoplastic findings in the pretreatment forceps biopsy. This resulted from either the complete removal of the lesion via forceps biopsy, or the misinterpretation of one of these samples. More than half of the NPD lesions after ER were diagnosed as PD after reviewing the ER slides. Therefore, we suggest that PD could partly explain the frequent occurrence of NPD after ER, and the use of PD as a subtype of GED could reduce the diagnostic discrepancy between the initial forceps biopsy and ER specimens.
Regarding the discrepancy in pathologic diagnoses between pretreatment forceps biopsy and ER specimens, it has been reported that most cases are up-staged upon pathologic diagnosis of the ER specimen compared to the initial biopsy specimen, for example, from LGD to adenocarcinoma [6,7]. However, up-staging of the histopathological diagnosis between the forceps biopsy and ER specimens is not clinically significant. The histopathological result of the forceps biopsy cannot represent the exact nature of gastric neoplastic lesions because of their small size. Therefore, ER could be regarded as an improved diagnostic procedure for early gastric neoplasms [5]. With respect to the discrepancies between negative histopathological findings from forceps biopsy and ER specimens, the frequency of NPD after ER is reported to be 3.2% to 4.4% in previous studies [8,19,20], which is comparable to the 2.5% of cases in our study. They also found that the most common initial pretreatment biopsy diagnosis was LGD, which is in agreement with our data showing a higher prevalence of LGD. However, in their study, there were no comments on PD, probably because the concept of gastric PD only appeared in the literature in 2011 [14].
Dysplasia is defined as dysplastic changes over the full length of the pit and the surface epithelium. However, several studies have demonstrated that epithelial dysplasia with surface maturation occurs in the esophagus [12,13] and stomach [14-16]. Previous reports investigating PD have mostly focused on the adjacent mucosa of resected gastric cancer tissue, showing variable frequencies and clinicopathological characteristics [14,16]. Regarding the biopsy features of PD, there have been several studies reporting similar findings, such as immature intestinal metaplasia or intestinal metaplasia with basal gland atypia [15,16,21]. Tava et al. [21] reported that hyperproliferative intestinal metaplasia has previously been categorized as a gastric indefinite lesion, and that 86% of cases were associated with concurrent gastric dysplasia or carcinoma. These lesions showed well-demarcated foci of metaplastic intestinalized glands with low-grade architectural disarray, exhibiting back-to-back architecture and an increased (moderate-to-high) mitotic activity [21]. Li et al. [15] also reported that intestinal metaplasia with basal gland atypia was present in 2.8% of gastric biopsies diagnosed as intestinal metaplasia or gastric dysplasia, and Agoston and Odze [16] reported that of 166 patients with intestinal metaplasia in their index gastric biopsies, 24 (14%) had PD-like atypia, and 25% progressed to conventional LGD in the follow-up. Taken together, these previous reports suggest that PD could be a preneoplastic lesion of gastric adenocarcinoma. From a practical point, PD lesions can be diagnosed as a type of GED or regenerative atypia in routine clinical practice.
There have been no studies describing the endoscopic features of PD until now. In the present study, although we did not directly compare the endoscopic features of PD with those of LGD or HGD, we tried to identify the endoscopic features of PD based on 16 lesions finally diagnosed as PD after ER. Approximately two-thirds of the PD lesions showed an elevated shape with a similar color to that of the surrounding normal mucosa. Therefore, when analysis of ER specimens shows negative pathology in a lesion initially diagnosed as a neoplasia, especially LGD, and if endoscopy has revealed an elevated lesion with a similar color to that of the surrounding normal mucosa, the possibility of PD should be considered.
To our knowledge, this is the first study to evaluate the significance of PD in NPD lesions after ER. However, our study has some limitations that can be addressed in future work. First, potential selection biases may have been present because of the retrospective nature of the study. ER was performed on a case-by-case basis according to clinical judgment and patient factors. Second, even though we tried to exclude the mistargeted cases in which ER might have been performed at the wrong site via a thorough review of the endoscopic images during ER as well as pre-ER endoscopic images, we may not have excluded all of these cases. However, all patients, except one who had a definitive final NPD, were followed up for at least 6 months, and there was neither recurrence at the previous resection site nor any new neoplastic lesions except one metachronous early gastric cancer. Therefore, it seems unlikely that mistargeted cases would have been included in the present study. Lastly, our study had a relatively small number of patients. If we could have included the data for cases in which the pretreatment forceps biopsy slides were not available, our results might have been different. Therefore, a further large-scale study involving a greater number of patients will be needed to confirm our results.
In conclusion, we found that approximately half of the lesions initially interpreted as NPD after ER could in fact be diagnosed as PD after review. In addition, some lesions were identified as LGD or HGD based on the initial biopsy specimen, although this initial diagnosis was also defined as PD after review. These diagnostic differences might be due to the tangential sectioning of biopsy specimens, making it difficult to identify the exact orientation of the gastric mucosa and surface maturation of gastric dysplastic lesions. Therefore, exact orientation of the biopsy specimen is crucial to diagnose PD in the stomach, and recognition of PD as one of subtypes of GED can reduce the diagnostic discrepancy between the initial forceps biopsy and ER specimens.
KEY MESSAGE
1. Negative pathologic diagnosis (NPD) after endoscopic resection (ER) resulted from either the complete removal of the lesion via forceps biopsy, or the misinterpretation of the samples.
2. More than half of the NPD lesions after ER were diagnosed as pit dysplasia (PD) after review.
3. PD can partly explain the frequent occurrence of NPD after ER, and the use of PD can reduce the diagnostic discrepancy between the initial forceps biopsy and ER specimens.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry for Health, Welfare and Family Affairs, Republic of Korea (0920050).