Clinical significance of changes in the corrected QT interval in stress-induced cardiomyopathy
Article information
Abstract
Background/Aims:
Although transient changes in the electrocardiogram (ECG) of patients with stress-induced cardiomyopathy (SCMP) are common, there are little data about ECG changes in patients with SCMP and the clinical implications of these variations.
Methods:
We investigated a total of 128 patients (age, 63.2 ± 15.4 years; female, 60.9%) diagnosed with SCMP. We compared the ECGs taken after SCMP diagnosis and during the recovery phase to those taken before SCMP diagnosis under baseline conditions. All patients were divided into two groups according to corrected QT (QTc) interval changes: recovered QTc group (QTc in SCMP > QTc in recovery phase, n = 77) and nonrecovered QTc group (QTc in SCMP ≤ QTc in recovery phase, n = 51).
Results:
In comparison of baseline, SCMP, and recovery phase, we found the mean heart rate (81.5 ± 18.7, 96.8 ± 25.3, and 83.0 ± 19.4/min, respectively; p < 0.001), frequencies of ST segment elevation (0.0%, 8.6%, and 1.6%, p = 0.004), ST segment depression (0.0%, 6.3%, and 1.6%, p = 0.007), T wave inversion (4.4 %, 43.8%, and 61.7%, p < 0.001), and QTc (447.4 ± 35.3, 488.9 ± 67.1, and 468.0 ± 49.5, p < 0.001) showed significant changes. In-hospital mortality (9.1% vs. 25.5%, p = 0.012) and critical care (54.5% vs. 72.5%, p = 0.040) occurred more frequently in the nonrecovered QTc group than in recovered QTc group.
Conclusions:
The QTc can be prolonged in patients with SCMP. Short-term mortality was increased in patients where the QTc did not recover.
INTRODUCTION
Stress-induced cardiomyopathy (SCMP), also known as apical ballooning syndrome or Takotsubo cardiomyopathy, is a cardiac syndrome characterized by transient left ventricular dysfunction that can mimic acute myocardial infarction (AMI) without obstructive coronary artery disease [1-3]. Almost two thirds of patients with SCMP experience an emotionally or physically stressful event [3,4] which immediately precedes the cardiac event. SCMP is associated with regional wall motion abnormalities involving hypokinesia or akinesia of the mid and apical segments of the left ventricle, and these wall motion abnormalities typically resolve themselves within several days [5].
Previous reports have demonstrated that patients with SCMP have various abnormalities in their electrocardiogram (ECG) such as ST-segment elevation, T wave inversion, nonspecific ST-T abnormalities, and prolongation of the corrected QT (QTc) interval [6-8]. Critically, repolarization dispersion, such as the prolongation of QTc interval, was also seen in the acute and subacute phases of SCMP [9]. The QTc interval is longer in SCMP than in AMI, and typically resolves over 3 to 4 months [9,10]. The prolonged QTc intervals are at risk of ventricular arrhythmia, such as polymorphic ventricular tachycardia, in patients with acute phase of SCMP [11-13]. Although these transient electrocardiographic changes are common features in patients with SCMP, there are little data about the serial electrocardiographic changes in patients with SCMP during hospitalization. Furthermore, the clinical implications of the QTc interval prolongation in SCMP patients have not been fully investigated. Therefore, the aim of this study is to investigate the clinical significance of QTc interval changes in patients with SCMP.
METHODS
Study design and patient selection
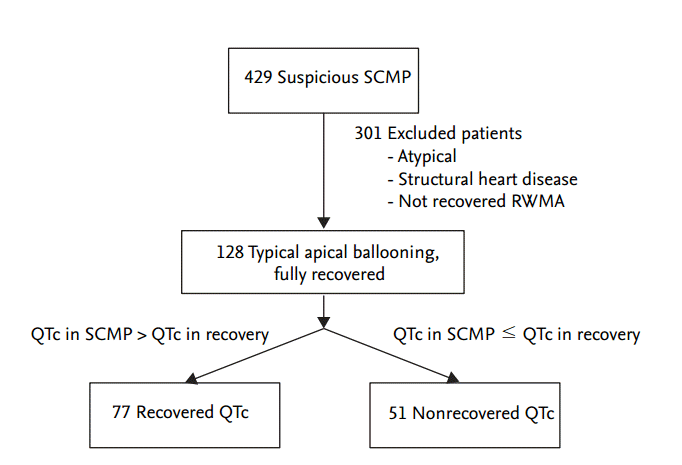
We retrospectively analyzed the clinical data of 429 patients with a clinical diagnosis of SCMP between February 2009 and March 2014 at a single university hospital. We excluded patients with atypical ballooning types (transient hypokinesis, akinesis, or dyskinesis of the left ventricular mid segments without apical involvement), structural heart disease, and regional wall motional abnormalities that were not recovered. Our study population consisted of 128 patients who had typical apical ballooning with no lasting wall motion abnormalities. The Institutional Review Board of Severance Cardiovascular Hospital waived the requirement for informed consent for this retrospective analysis. Fig. 1 outlines the patient selection process.
Diagnosis of SCMP
All patients fulfilled the Mayo Clinic criteria for SCMP [1,14], and follow-up echocardiograms demonstrated complete resolution of the left ventricular dysfunction. We categorized the time course of SCMP as the baseline phase, the SCMP diagnosis phase, and the recovery phase. Each phase was defined by the following methods: (1) the baseline phase was defined as a clinical period before the presence of any cardiac dysfunction or abnormal cardiac events, (2) the SCMP diagnosis phase was defined as clinically diagnosed SCMP with the lowest ejection fraction (EF) assessed by echocardiogram, and (3) the recovery phase was defined as a clinical recovery with fully recovered left ventricular function assessed by echocardiogram. We evaluated ECG parameters during each of these phases. The wall motion score index (WMSI) was calculated by the average of all analyzed segment scores.
Electrocardiogram analysis
We evaluated all 128 study subjects’ serial ECGs according to their serial echocardiographic findings, and we obtained the baseline ECG of 113 patients (88.3%). Twelve-lead ECGs were recorded at a paper speed of 25 mm/sec with a 10 mm/mV amplification scale using the GE Marquette MUSE system (GE Medial System, Milwaukee, WI, USA). The high and low cut-off frequencies were 100 and 0.5 Hz, respectively. The ECGs were simultaneously assessed by two investigators who were blinded of clinical characteristics, patients group, and in-hospital course.
We compared the serial ECG changes of each patient taking during each phase of their hospitalization: the baseline phase, the SCMP diagnosis phase, and the recovery phase. We acquired the following ECG parameters: heart rate, ST elevation (> 1 mm in at least two contiguous leads), ST depression (> 1 mm in at least two contiguous leads), and T wave inversion (inverted T wave amplitude > 3 mm in at least three precordial or inferior leads). The QTc interval (corrected for heart rate according to Bazett’s formula, QTc = QT/√RR) was automatically measured. We defined an abnormally prolonged QTc interval as > 450 ms in men and > 470 ms in women [15]. All patients were divided into two groups according to QTc interval changes: (1) recovered QTc group (QTc in SCMP > QTc in recovery phase, n = 77), or (2) nonrecovered QTc group (QTc in SCMP ≤ QTc in recovery phase, n = 51). We excluded QTc prolongation that was induced by drugs and electrolyte imbalance.
Study endpoints
The primary endpoint was in-hospital all-cause mortality after study patients were diagnosed with SCMP. The secondary endpoints were incidences of critical care after SCMP diagnosis and the length of hospital stay. Critical care was defined as dependence on ventilator care or infusion of inotropes.
Statistical analysis
Statistical analysis was performed using SPSS version 20.0.0 (IBM Co., Armonk, NY, USA). Continuous variables were compared by an independent t test after normality tests. Not normally distributed continuous variables were compared by the Mann-Whitney U test. Data are expressed as number (%) or as mean ± standard deviation or median (interquartile range). Comparisons of categorical data were made using chi-square statistics or Fisher exact test. Changes in ECG parameters of each patient were compared with the use of generalized-estimating-equation models (for categorical outcomes) and repeated measures linear mixed-effects models (for continuous outcomes). In-hospital mortality was analyzed by Kaplan-Meier survival curves, and the differences between cumulative in-hospital mortality rates were compared with the log rank test. Multivariate logistic analysis was performed to determine the independent risk factors. The variables with a p < 0.05 in the univariate analysis and traditional cardiac risk factors (age and gender) were included in the multivariate regression model. A p < 0.05 was considered statistically significant.
RESULTS
Baseline characteristics of the study population
One hundred and twenty-eight patients were included in this study. The patients ranged in age from 15 to 90 years (mean age, 63.2 ± 15.4), and were 60.9% (78/128) female (Table 1). The most common trigger factor of SCMP was physical stress (89.1%, 114/128). Pre-existing critical illness, such as sepsis, respiratory insufficiency, major noncardiac surgery, or cerebrovascular accident, were similar between two groups. The median time interval from SCMP diagnosis to recovery phase was 13.0 days (interquartile range [IQR], 7.0 to 32.0) and similar in the two groups (15.0 days [IQR, 7.0 to 38.0] in the recovered QTc group and 10.0 days [IQR, 7.0 to 23.0] in the nonrecovered QTc group, p = 0.199). Pharmacological treatment such as β-blocker or calcium channel blocker, potentially affecting the QTc interval and the arrhythmogenic risk, were similar between two groups.
Serial changes of echocardiographic and electrocardiographic parameters
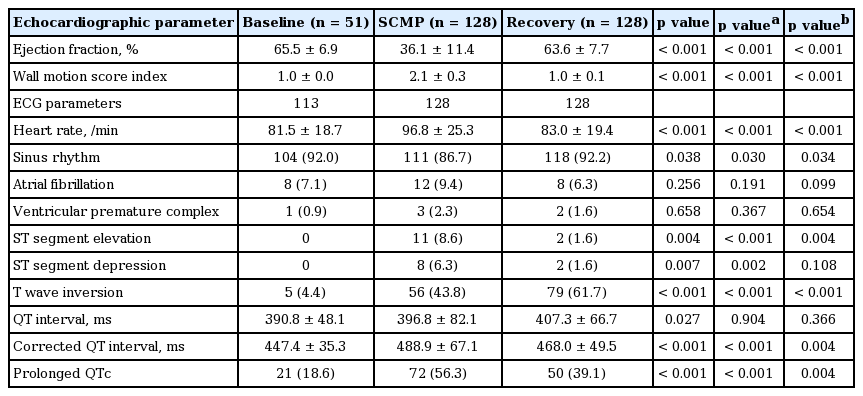
Baseline echocardiography of 51 patients and the baseline ECG of 113 patients were analyzed. Left ventricular EF was markedly decreased during the SCMP diagnosis phase compared to both baseline and the recovery phase (65.5% ± 6.9% vs. 36.1% ± 11.4% vs. 63.6% ± 7.7%, respectively; p < 0.001) (Table 2). Heart rate was also significantly elevated during the SCMP diagnosis phase (81.5 ± 18.7/min vs. 96.8 ± 25.3/min vs. 83.0 ± 19.4/min, respectively; p < 0.001). ST elevation (0.0% vs. 8.6% vs. 1.6%, respectively; p = 0.004) and ST depression (0.0% vs. 6.2% vs. 1.6%, respectively; p = 0.007) were more frequently observed in the SCMP phase compared to either the baseline or recovery phases. However, T wave inversion was more frequently observed in the recovery phase than either the baseline or SCMP phase (4.4% vs. 43.8% vs. 61.7%, respectively; p < 0.001). The QTc interval was significantly prolonged during the SCMP phase (447.4 ± 35.3 ms vs. 488.9 ± 67.1 ms vs. 468.0 ± 49.5 ms, respectively; p < 0.001), and returned to lengths observed in baseline after echocardiography showed recovery of left ventricular systolic function. The prevalence of QTc prolongation was also more frequently observed in the SCMP phase than the other phases (18.6% vs. 56.3% vs. 39.1%, respectively; p < 0.001).
Comparison of clinical, echocardiographic, and electrocardiographic characteristics between recovered vs. nonrecovered QTc group
Even though the average QTc interval returned to baseline levels for all patients, the simultaneous recovery of left ventricular systolic function with an attenuated QTc interval was not observed in all patients. Of all the patients in this study, 77 patients were categorized into the recovered QTc group (QTc in SCMP > QTc in recovery phase) (Fig. 2), and 51 patients were categorized into the nonrecovered QTc group (QTc in SCMP ≤ QTc in recovery phase) (Fig. 3). The baseline characteristics of these two groups were not significantly different (Table 1). Echocardiography showed that there were no differences in left ventricular EF at baseline or recovery between the two groups. However, the nonrecovered QTc group showed a lower EF compared to the recovered QTc group (33.5% ± 11.2% and 37.8% ± 11.3%, respectively; p = 0.034) during the SCMP diagnosis phase. The WMSI was 2.08 ± 0.31 when study patients were diagnosed with SCMP, and there was no statistical difference between two groups, even if their left ventricular systolic function fully recovered.

The echocardiography and electrocardiogram of 64-years old male presenting stress-induced cardiomyopathy. He was categorized as the recovered corrected QT (QTc) group. (A) The baseline phase, (B) the stress-induced cardiomyopathy diagnosis phase, (C) the recovery phase. Arrowheads indicated the typical apical ballooning. EF, ejection fraction; HR, heart rate.

The echocardiography and electrocardiogram of 89-years old female presenting stress-induced cardiomyopathy. She was categorized as the nonrecovered corrected QT (QTc) group. (A) The baseline phase, (B) the stress-induced cardiomyopathy diagnosis phase, (C) the recovery phase. Arrowheads indicated the typical apical ballooning. EF, ejection fraction; HR, heart rate.
The heart rate during the SCMP diagnosis phase of the nonrecovered QTc group was faster than that of the recovered QTc group (103.5 ± 27.9/min vs. 92.3 ± 22.5/min, p = 0.014), but the frequencies of ST elevation, ST depression, and T wave inversion showed no significant differences between the two groups. There was a significant difference in QTc during both the SCMP diagnosis and recovery phases when comparing the recovered and nonrecovered QTc group (522.9 ± 53.2 ms vs. 437.7 ±51.9 ms, p < 0.001 at SCMP diagnosis; 456.3 ± 45.6 ms vs. 485.6 ± 50.3 ms, p = 0.001 at recovery).
Nonrecovered QTc interval is predictor for in-hospital mortality
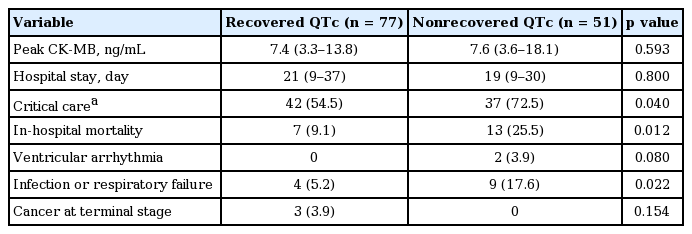
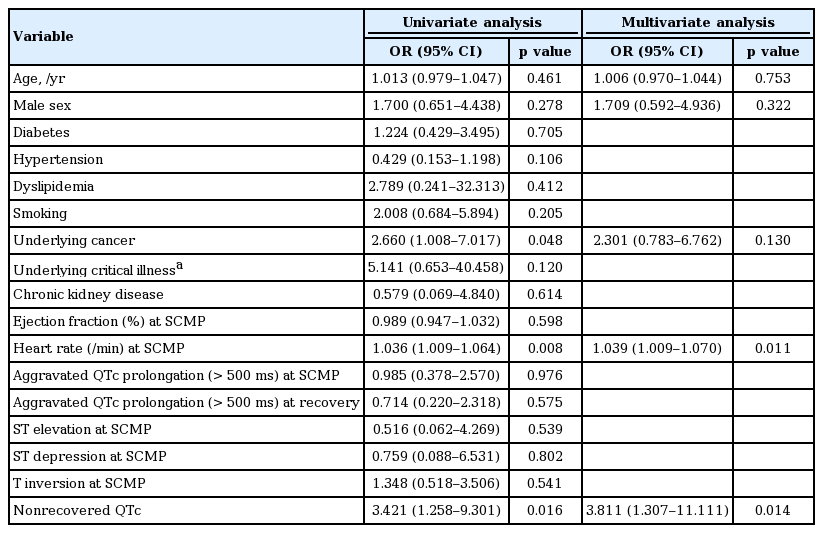
There was no difference between two groups with regard to peak creatinine kinase-MB values and the length of hospital stay. In-hospital all-cause mortality occurred more frequently in the nonrecovered QTc group than in the recovered QTc group (25.5% vs. 9.1%, p = 0.012) (Table 3, Fig. 4). Severe sepsis and respiratory failure were the major causes of death in nonrecovered QTc group (17.6% vs. 5.2%, p = 0.022). The incidence of critical care was also more frequently observed during hospitalization in the nonrecovered QTc group than in the recovered QTc group (72.5% vs. 54.5%, p = 0.040) (Table 3). In univariate logistic regression analysis, underlying cancer (odds ratio [OR], 2.660; 95% confidence interval [CI], 1.008 to 7.017; p = 0.048) (Table 4), heart rate at SCMP (OR, 1.036; 95% CI, 1.009 to 1.064) and nonrecovered QTc (OR, 3.421; 95% CI, 1.258 to 9.301) were associated with increased risk of in-hospital mortality. Using multivariate logistic regression analysis, the significant determinant for in-hospital mortality was nonrecovered QTc (OR, 3.811; 95% CI, 1.307 to 11.111; p = 0.014), along with heart rate as a predictor of in-hospital mortality (OR, 1.039; 95% CI, 1.009 to 1.070; p = 0.011).

Kaplan-Meier for cumulative in-hospital mortality incidence (median follow-up duration, 19.5 days; p = 0.012 by log-rank). QTc, corrected QT.
All 20 in-hospital mortality cases occurred upon physical stress, and these patients had near complete recovery of left ventricular EF. Two of these patients had developed sudden cardiac arrest, and it was highly suspected that these patients suffered from ventricular tachycardia. These patients were also were categorized into the nonrecovered QTc group.
DISCUSSION
The major findings of this study are that (1) ECG parameters of ST segment changes, or QTc interval could indicate whether left ventricular dysfunction present at the time of SCMP diagnosis was recovered or not, and (2) patients whose QTc interval did not recover to baseline levels during the SCMP recovery phase had a higher rate of in-hospital mortality or critical care.
Transient changes in the ECG of patients with SCMP are commonly observed. A previous study reported the time course of these ECG changes and demonstrated a deepened T wave inversion and a doubling of the QTc interval in SCMP patients [10]. Our findings are consistent with this report. We extended these findings by matching ECG parameters with the time course of echocardiographic changes when possible. This allowed us to demonstrate that both ST elevation or depression normalization and T wave inversions co-varied with recovery from left ventricular dysfunction. Systolic dysfunction and the regional wall motion abnormalities usually return to normal within several weeks [5,6,16]. Currently, the recovery of left ventricular dysfunction is typically evaluated by echocardiography only, but our data suggests that ECG parameters such as ST change, T wave properties, or the QTc interval may also be useful to help determine if recovery from SCMP has occurred.
In our study, when left ventricular EF had recovered in patients with SCMP, we found that 77/128 patients (60.2%) presented with a recovered QTc interval, and 51/128 patients (39.8%) presented with a still prolonged QTc interval. Although most of the clinical parameters were similar between the two groups, patients in the nonrecovered QTc group showed a lower left ventricular EF at the time of SCMP diagnosis and were more likely to be associated with in-hospital mortality. While a history of cancer was one of the risk factors for in-hospital mortality in univariate analysis, only nonrecovered QTc interval was an independent risk factor for in-hos pital mortality. Previous report showed that pre-existing critical illness, such as sepsis, respiratory insufficiency, major noncardiac surgery, or cerebrovascular accident, were related with higher in-hospital mortality rate [17]. Our study suggested that pre-existing critical illness was not sufficient to significantly increased in-hospital mortality. We may also consider electrocardiographic changes including QTc in patient with SCMP.
The predictive value of QT dispersion for adverse outcomes has been evaluated in populations with myocardial infarction, and QT dispersion was demonstrated as a predictive factor for malignant ventricular arrhythmia [18,19]. Previous studies also demonstrated that the QT interval might be influenced by the extent of myocardial damage in patients with chronic myocardial infarction [20]. A previous report demonstrated that SCMP is rarely associated with malignant ventricular arrhythmia, even though both a significantly long QT interval and repolarization dispersion were observed [9]. However, in 2005 Denney et al. [11] reported a case of SCMP with QT interval prolongation in a young man who developed torsade de pointes and it now can carry a small but significant lethal risk for adverse outcomes, including cardiac mortality [21]. Cho et al. [13] also reported a case of SCMP presenting as VT as an initial manifestation. In our study, only two of the 20 in-hospital mortality cases (10%) had developed sudden cardiac arrest. Both these patients suffered from malignant ventricular arrhythmia at the time of death, suggesting that this might be one of the mechanisms responsible for the observed in-hospital mortality. Furthermore, severe form of septic shock and respiratory failure were the major causes of death in nonrecovered QTc group. It is also hypothesized that severe extensive inflammatory reaction accompanying catecholamine surge with myocardial dysfunction can be related with continued repolarization abnormality.
Although overall in-hospital mortality from SCMP is very low, in the subgroup of patients where a physical stressor was present, such as illness or major surgery, prognosis was worse [4]. A recent study divided patients with SCMP into three groups according to ECG abnormalities at presentation such as ST elevation, T wave inversion, or non-specific ST-T abnormalities, but these abnormalities did not correlate with clinical outcomes [8]. That study categorized three groups according to ECG findings present only at SCMP presentation, but our study analyzed serial ECG changes during hospitalization, and demonstrated that a nonrecovered QTc interval was correlated with adverse outcomes. Our study is the first study using serial ECG and echocardiographic changes, and our data is the first risk predicting model for patients diagnosed with SCMP. In other words, by analyzing several ECG parameters of patient with SCMP, we can predict the patient’s clinical phase of SCMP and in-hospital outcomes.
This study had several limitations. First, it was a retrospective study based on medical records. Further prospective studies are required to examine the significance of QTc interval changes. Second, we included only patients with SCMP who showed fully recovered left ventricular EF by echocardiography during the recovery phase. This may have potential for selection bias. Therefore, these results are only applicable to patients with follow-up echocardiography. Third, not all study populations performed coronary angiography for the purpose of excluding left ventricular dysfunction due to coronary artery disease. In our study, only 46 patients (35.9%) underwent coronary angiography. However, we only included typical apical ballooning that fulfilled with Mayo Clinic criteria for SCMP and fully recovered, and we excluded patients with possible ischemic heart disease. Fourth, not all baseline ECGs or echocardiographic data of each patient were available. However, by using linear mixed-effects models and generalized-estimation-equation models, we can analyze all serial ECGs and echocardiographic data of each patient.
Some ECG parameters can be a surrogate marker of the clinical outcomes of patients presenting with SCMP. Even in SCMP patients who had complete recovery of left ventricular systolic function, a continued QTc prolongation was an independent risk factor for in-hospital mortality.
KEY MESSAGE
1. Electrocardiogram parameters, such as ST segment changes, or corrected QT (QTc) interval, could be useful to determine whether left ventricular dysfunction was recovered or not.
2. A continued QTc prolongation can be associated with increased risk of in-hospital mortality in patients with stress-induced cardiomyopathy.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
The authors would like to acknowledge the contribution of the members of the Department of Research Affairs, Biostatistics Collaboration Unit, Yonsei University College of Medicine: Yun Ho Roh, M.S, Bo Gyoung Ma, M.S.