A neurometabolite study of chronic daily headache in patients with systemic lupus erythematosus using magnetic resonance spectroscopy: comparison with fibromyalgia patients and healthy controls
Article information
Abstract
Background/Aims
Neuropsychiatric systemic lupus erythematosus (SLE) includes a broad spectrum of neurologic and psychiatric manifestations. One of the most commonly observed neuropsychiatric symptoms is headache. However, the lack of specific clinical distinctions for headache in SLE has made it difficult to elucidate its pathophysiology. The aim of this study is to evaluate the neurometabolic changes using Proton Magnetic Resonance Spectroscopy (1H-MRS) in patients with SLE who suffer from chronic daily headache (CDH).
Methods
SLE and fibromyalgia patients with CDH and healthy controls were recruited (n = 9, n = 5, and n = 6, respectively). 1H-MRS metabolite ratios were evaluated in bilateral basal ganglia (BG) and bilateral peritrigonal white matter (PWM).
Results
1H-MRS showed a significantly decreased N-acetylaspartate (NAA)/creatine (Cr) ratio in right BG in SLE patients with CDH compared to fibromyalgia patients with CDH and normal controls (p = 0.029 and p = 0.020, respectively). Left PWM NAA/Cr and choline/Cr ratios in SLE patients with CDH were lower than those in fibromyalgia patients with CDH (p = 0.019 and p = 0.029, respectively).
Conclusions
This study suggests the possibility that CDH in patients with SLE might be associated with neuronal dysfunction and neurometabolic changes.
INTRODUCTION
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease mainly affecting the young age group including women of childbearing age. Approximately 2/3 of SLE patients have neuropsychiatric symptoms [1]. Neuropsychiatric systemic lupus erythematosus (NPSLE) includes a broad spectrum of neurologic and psychiatric manifestations. Headache is one of the most common symptoms of SLE patients but the relatedness to SLE is still controversial [2]. Chronic daily headache (CDH) is defined as a primary headache occurring at least 15 days per month and it is one of the most common problems in a headache clinic [3]. CDH patients do not respond well to medical treatment and take medical services repetitively; hence, curing CDH is very important from a therapeutic viewpoint.
Proton Magnetic Resonance Spectroscopy (1H-MRS) is a non-invasive test that can demonstrate changes in neuronal metabolites [4]. In recent years, many studies using 1H-MRS have been actively conducted in NPSLE patients and the recommendation for NPSLE management proposed by the European League Against Rheumatism (EULAR) stated that if conventional magnetic resonance imaging (MRI) is normal and signs or symptoms cannot be explained, 1H-MRS can be considered [4,5]. However, most studies used 1H-MRS to compare patients with minor NPSLE versus patients with major NPSLE or they compared the SLE patients according to the existence of NPSLE [4]. However, no study has been conducted using MRS with a focus on specific symptoms, such as headache. Therefore, we aimed to evaluate neurometabolic changes by using 1H-MRS in SLE patients with CDH, and to compare them with fibromyalgia patients with CDH, or healthy controls.
METHODS
Subjects
SLE patients with CDH, fibromyalgia syndrome (FMS) patients with CDH, and healthy controls who had no organic brain lesion were recruited into this study. The study participants with CDH were enrolled from among the patients who were registered at the Rheumatology Clinic of Keimyung University Dongsan Medical Center in Daegu. If the subject presented with headache for more than 15 days per month for longer than 3 months, he/she was defined as having CDH. A total of nine SLE patients without history of neuropsychiatric symptoms or current neuropsychiatric symptoms were Koreans and they fulfilled the 1997 American College of Rheumatology (ACR) revised criteria for the classification of SLE [6]. Furthermore, patients who did not have secondary FMS and any other comorbidity or history of medication that could cause a headache were selected. We reviewed data obtained from the subjects according to age, gender, concomitant treatment, disease duration, antinuclear antibody, extractable nuclear antigen antibody, and anti-phospholipid antibody. The patients underwent required blood tests for assessment of the disease activity by the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) [7].
To compare the MRS among SLE patients, FMS patients, and healthy controls, we recruited five age- and sex-matched FMS patients and six age- and sex-matched healthy subjects as the control group. A total of five FMS patients were also Korean nationals, and they met the 2010 ACR criteria for FMS [8]. We recruited SLE and FMS patients with CDH who were aged 18 years or older and who had received regular treatment for 3 months prior to study enrollment. Patients who had diabetes, stroke, renal insufficiency, and alcohol or substance abuse were excluded from the study. Healthy controls who did not have any disease that could cause a headache and who were not taking any drugs were recruited into this study. This study used a cross-sectional design.
Proton Magnetic Resonance Spectroscopy
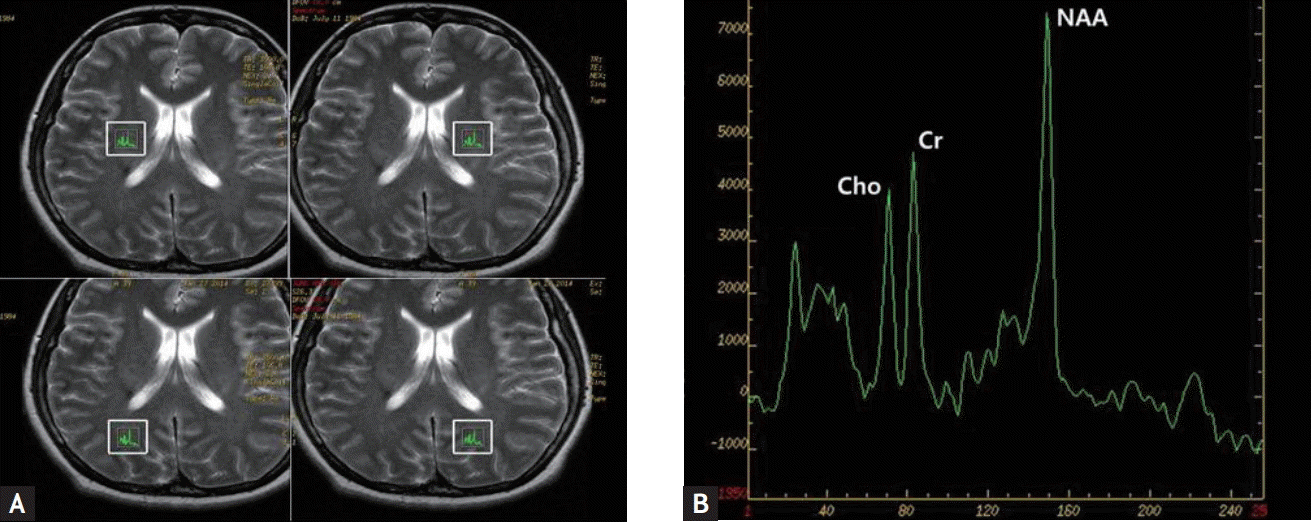
1H-MRS was performed with a single voxel technique using point-resolved spectroscopy sequence with the following parameters: repetition time = 1,500 ms, echo time = 30 ms, number of excitations = one, and 8 cm3 (2 × 2 × 2 cm) voxels of interest (VOIs). Four VOIs were right and left basal ganglia (BG) and right and left peritrigonal white matter (PWM) because these VOIs are the most selected areas in the previous studies [4]. The total parameter scan time was 3 minutes 42 seconds for each VOI. The metabolite concentrations of N-acetylaspartate (NAA), myoinositol (mI), choline (Cho), and creatine (Cr) were analyzed. The NAA/Cr, Cho/Cr, NAA/Cho, mI/Cr, and NAA/mI ratios were calculated (Fig. 1).
Statistical analysis
All statistical analyses were performed using SPSS version 20.0 (IBM Co., Armonk, NY, USA). Results are presented as mean ± SD unless specified otherwise. The non-parametric Kruskal-Wallis test was used for between-group comparisons. The Bonferroni correction was applied to multiple comparisons within each variable. p values less than 0.05 were considered statistically significant.
Ethics statement
This study was approved by the Institutional Review Board (IRB) of Keimyung University Dongsan Medical Center (IRB No. 2013-05-031). Written informed consent was obtained from all participants and confirmed by the board.
RESULTS
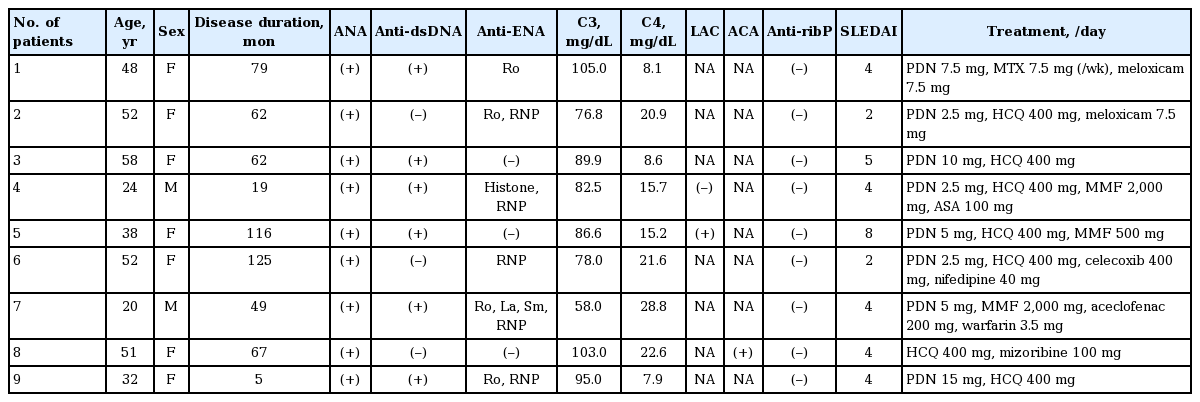
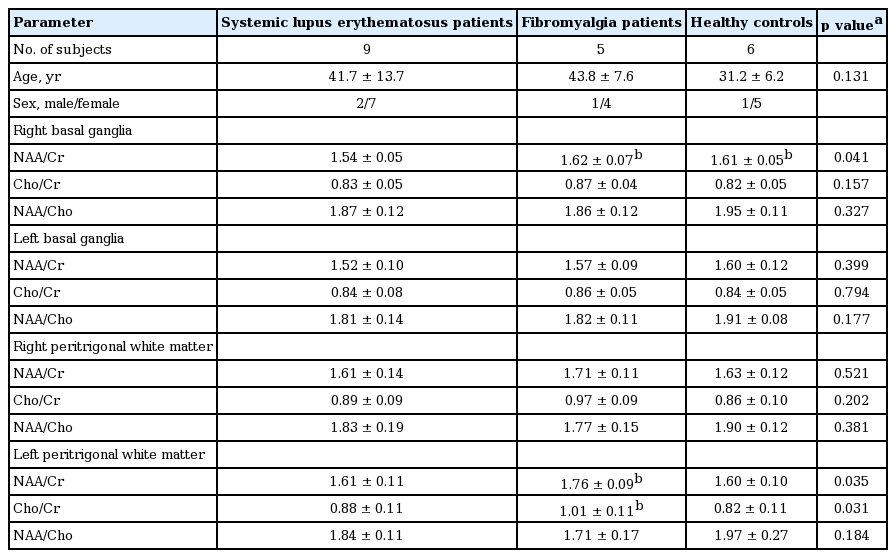
Table 1 presents the clinical and laboratory data of SLE patients enrolled in the study. Eight out of the nine SLE patients with CDH had SLEDAI < 6, indicating low disease activity. Two SLE patients had antiphospholipid antibody syndrome. Three out of the nine SLE patients had lupus nephritis. The median dose of corticosteroids was 5.6 mg/day. Eight patients were taking hydroxychloroquine, and two patients were taking mycophenolate mofetil. Table 2 shows the results of 1H-MRS measurements in SLE patients, FMS patients, and healthy controls. The mean age was not different among groups (41.7 ± 13.7, 43.8 ± 7.6, and 31.2 ± 6.2 years for SLE patients with CDH, FMS patients with CDH, and healthy controls, respectively). The NAA/Cr ratio in right BG of patients with SLE, FMS, and healthy controls was 1.54 ± 0.05, 1.62 ± 0.07, and 1.61 ± 0.05, respectively. Even after applying Bonferroni correction, the results showed a significantly decreased NAA/Cr ratio in right BG in SLE patients with CDH compared to fibromyalgia patients with CDH and normal controls (p = 0.029 and p = 0.020, respectively). However, there were no significant differences in the NAA/Cr ratio in left BG among the three groups. Left PWM NAA/Cr and Cho/Cr ratios in SLE patients with CDH were significantly lower than those in FMS patients with CDH (p = 0.019 and p = 0.029, respectively). Contrary to our thoughts, left PWM NAA/Cr and Cho/Cr ratios in FMS patients with CDH were significantly higher than those in healthy controls (p = 0.047 and p = 0.023, respectively). There were no significant differences in mI/Cr and NAA/mI ratios among the three groups (data not shown).
DISCUSSION
NPSLE consists of 19 syndromes including neurologic and psychiatric problems that affect the central nervous system (CNS) and the peripheral nervous system [1]. Among these problems, headache is the most common symptom which is observed in migraine, tension, benign intracranial hypertension, and non-specific intractable headache [9]. There is no standardized method to assess whether headache is related to lupus. Headache can occur due to secondary causes such as infection, medication, and hypertension [2]. In addition, it is controversial that headache in SLE patients can be considered as part of neurologic involvement and no correlation was found with disease activity [4].
Laboratory diagnosis of NPSLE is very difficult and many cases have no correlation with disease activity of SLE [10]. Cerebrospinal fluid examination, electroencephalography, and MRI can be helpful in diagnosing NPSLE. If MRI shows normal or nonspecific changes, a more advanced imaging modality should be considered. According to the recommendation for management of NPSLE proposed by the EULAR, a more advanced imaging modality refers to quantitative MRI, single photon emission computed tomography, and positron emission tomography [5].
1H-MRS has been applied in physiological, biochemical, and pathological studies of the brain as a clinical tool to measure the amount of various metabolites in tissues non-invasively [4,11]. The proportion between metabolites reflects the molecular and cytological process. The NAA level is correlated with neuronal and axonal density, and mI is considered as a marker of gliosis. Cho refers to myelin content and it reflects membrane turnover, while Cr is used as a reference metabolite. As observed in studies on SLE using 1H-MRS, the NAA level was decreased in both NPSLE patients and SLE patients when the Cho and mI levels were increased or decreased [4]. However, few studies analyzed the specific manifestations using 1H-MRS. Several studies showed that the Cho/Cr ratio was increased in patients with cognitive impairment [12,13].
In this study, 1H-MRS was conducted in patients who satisfied the definition of CDH [3]. In reality, it is difficult to recruit CDH controls without underlying diseases. FMS excluded generous organic causes of disease is often accompanied by headache. Because both FMS and CDH have pathophysiology for nonorganic chronic pain, we selected FMS patients with CDH as a control group. Our study result was consistent with other 1H-MRS studies conducted in other SLE patients. The results indicate that the NAA/Cr ratio in SLE patients was reduced more in the right BG compared to that in fibromyalgia patients and healthy controls. The metabolites in BG were measured in previous SLE studies [14,15]. Pragmatic trace metals are often deposited in the BG within the brain, and the BG are thought to be the most metabolically active part that is affected during hypoxic brain damage [15]. Furthermore, the NAA/Cr ratio was reduced more in the left PWM of SLE patients than fibromyalgia patients, but no significant difference was observed when compared to healthy controls. The reason for the change in metabolites in the white matter area was that the commissural tract and a large area of the association tract were damaged thereby causing neuropsychiatric symptoms of NPSLE [16]. However, further studies are needed for elucidating the difference of left PWM NAA/Cr and Cho/Cr ratio between fibromyalgia patients and healthy controls because our study includes only a limited number of patients.
Our study had some limitations. First, the test was conducted only in two areas locally: BG and PWM. In other SLE studies, a number of areas such as periventricular, occipital, and frontal regions of white matter as well as the insular area were studied [4,11,16,17]. It has been reported that there was no difference in metabolites in the white matter regions between normal subjects and FMS patients and the NAA level in the hippocampus was reduced according to the 1H-MRS studies [18-20]. Second, the present study could not assess whether there were any changes in metabolites after treatment of CDH due to its cross-sectional design. If prospective studies are conducted, changes in metabolites due to disease activity or treatment can be used as a marker. Third, it was difficult to recruit a large number of SLE and FMS patients with CDH due to low prevalence of CDH. Because of this limitation, we could not detect any differences according to the disease duration or treatment regimen. Therefore, a multicenter study should be performed so that a large number of patients can be recruited. However to date, no 1H-MRS studies have been conducted with respect to specific CNS manifestations such as CDH. Fourth, SLE patients without CDH were not enrolled in this study. We did not compare the metabolites between SLE patients with CDH and SLE patients without CDH.
Based on the results of this study, we expect that additional studies including many cases with CNS manifestations will be performed in the future. Our study results provide useful evidence to shed light on the 1H-MRS study of specific CNS manifestations of NPSLE and pathophysiology of CDH.
KEY MESSAGE
1. This study suggests the possibility that chronic daily headache (CDH) in patients with systemic lupus erythematosus might be associated with neuronal dysfunction and neurometabolic changes.
2. Our study results provide useful evidence to shed light on the Proton Magnetic Resonance Spectroscopy (1H-MRS) study of specific central nervous system manifestations of neuropsychiatric systemic lupus erythematosus and pathophysiology of CDH.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This work was supported by the research promoting grant from the Keimyung University Dongsan Medical Center in 2013.