An analysis of age-related loss of skeletal muscle mass and its significance on osteoarthritis in a Korean population
Article information
Abstract
Background/Aims:
This study was conducted in order to analyze the effects of sarcopenia on age-related osteoarthritis (OA) of the knee in a Korean population.
Methods:
All the Korean subjects who visited the Yeungnam University Medical Center Health Promotion Center between 2008 and 2012 in order to undergo a routine medical examination were enrolled. A total of 5,723 young, healthy people (2,959 males, 2,764 females) enrolled as normal subjects and 23,473 subjects (13,006 males and 10,467 females) were included for evaluation of the effects of sarcopenia on OA. There were 266 subjects who followed-up bioelectrical impedance analysis at a 4-year interval. Of 327 subjects enrolled in this study, knees with anteroposterior X-rays were assessed according to the Kellgren-Lawrence (K/L) grade.
Results:
Skeletal muscle mass index (SMI) and basal metabolic rate (BMR) showed a steady decrease with the advance of age (p < 0.01), but SMI showed strong positive correlation with BMR (r = 0.72, β = 30.96, p < 0.01). During the 4-year interval, BMR showed a significant decrease with aging (p < 0.01), consistently with the decrease of SMI. Knees with normal SMI were prone to be designated as K/L grade 0 or 1; however, subjects with sarcopenia showed a trend toward the higher K/L grade, classified as knee radiological osteoarthritis (ROA) (p < 0.01).
Conclusions:
The results of this study may indicate that sarcopenia as age-related loss of skeletal muscle mass is interactively correlated with the presence and severity of age-related OA.
INTRODUCTION
Age-related osteoarthritis (OA) is characterized by degradation of articular cartilage and substantial loss of matrix [1], as a consequence of senescence. The symptoms and signs usually appear after the fifth decade [2], and approximately 68% of women and 58% of men older than 65 years of age have OA [3]. Intra-articular cell senescence and cartilage matrix degradation [4], extra-articular loss of skeletal muscle mass (SMM) [5] and deteriorated proprioception [6] contribute to development of OA. In addition to age, obesity is regarded as another major risk factor for OA, especially in the elderly [7].
Body composition refers to the mass percentages of muscle, fat, and bone, as well as water of the body, and the spaces taken up by the compartments determine leanness/obesity of the body. The aging process accompanies altered body composition [8] with osteoporosis [9] as the normal consequences of cellular apoptosis [10], mitochondrial dysfunction [11], neurohormonal changes [12], nutritional insufficiency [13], and increasing pro-inflammatory cytokine levels [14] in association with decreasing physical activity and performance [15]. Age-related degenerative loss of SMM and strength is referred to as sarcopenia, which can cause an increase in the percentage of body fat (PBF) mass as sarcopenic obesity [8,9,11-13]. SMM is the metabolically active body component; however, fat mass (FM) is metabolically inactive, so that the changes of SMM and FM with aging can be one of the most relevant biomarkers of senescence [16]. In general, the body mass index (BMI) is used for determination of obesity, a measure of body fat based on height and weight which applies to adults [17-23]. However, if obesity is defined as the gaining of FM in the aspect of metabolic alteration, the SMM and PBF could be a more critical parameter than BMI. Baumgartner et al. defined sarcopenia as the appendicular skeletal muscle mass (ASM) divided by body height squared in meters, which is –2 or lesser standard deviations (SD) below the reference values for young, healthy individuals of the same sex and ethnicity [24,25]. Janssen et al. [26] proposed percentage-skeletal muscle mass index (SMI), absolute SMM (kg) to percentage of weight (muscle mass/body mass × 100), and defined sarcopenia as a SMI less than one SD below the reference values of bioelectrical impedance analysis (BIA) measured from sex- and ethnicity-matched healthy individuals between the ages of 18 and 39 years. A SMI between –1 to –2 SD of the mean is referred to as class I sarcopenia, and a SMI below –2 SD of the mean as class II sarcopenia [26].
This study was conducted in order to analyze the changes of SMM and body composition in association with aging, and to evaluate the effects of sarcopenia and SMI on age-related knee OA in a Korean population.
METHODS
Procurement of study populations
All the Korean subjects (age 18 and older) who visited the Yeungnam University Medical Center Health Promotion Center (Daegu, Korea) between 2008 and 2012 in order to undergo a routine medical examination, including BIA, were enrolled in this study. Any cases of erythrocyte sedimentation rate ≥ 40 mm/hr, positive rheumatoid factor, abnormal results on laboratory tests and a chest X-ray, and a medical history of diabetes, malignancies, joint trauma or surgery, and other metabolic or inflammatory diseases were excluded. A total of 5,723 normal subjects (2,959 males, 2,764 females) aged between 18 and 39 years were enrolled as the young and healthy Korean population, and 23,473 subjects (13,006 males and 10,467 females) were included for evaluation of the effects of sarcopenia on OA. For analysis of the effect of aging on body composition, 266 subjects who followed-up BIA at a 4-year interval from 2008 to 2012 were evaluated regarding the interval changes in paired comparison. The study was conducted according to the code of ethics of the World Medical Association (Declaration of Helsinki) and all of the procedures followed were approved by the Ethical Committee of the Yeungnam University Medical Center.
Determination of body mass and body composition by BIA
Body weight, height, BMI, basal metabolic rate (BMR), FM, PBF, ASM, SMM, and SMI were determined using standardized equipment and procedures. BMR was calculated using the Cunningham equation, BMI as weight/height2 (kg/m2), SMI as ASM/body mass × 100 (%), and PBF as FM/body mass × 100 (%). For measurement of body composition, dual energy X-ray absorptiometry (DXA) is the standard for clinical analysis; however, due to its reliability and convenience, BIA can replace DXA [27]. The body components were assessed by BIA using InBody 720 (Biospace, Seoul, Korea). In principle, BIA is based on the height and impedance value of the body and body water volume on BIA determines fat and fat-free mass of the body. From the data, sarcopenia was defined as a weight-adjusted SMI below –1 SD of the reference values. Analysis of BIA data was performed for comparison of the effects of sarcopenia on age-related OA in the populations.
Evaluation of radiological osteoarthritis and Kellgren-Lawrence grade of the knee
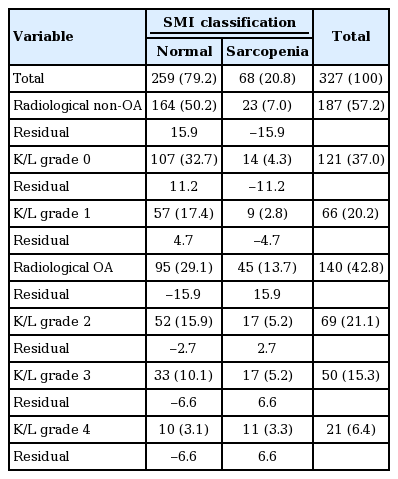
The American College of Rheumatology (ACR) criteria for knee OA are comprised of age criterion, joint symptoms, exclusion of inflammatory conditions, and positive radiography of the knee [28]. The significances of SMI were analyzed in the aspect of aging and age-related OA. Radiological bony changes were graded on anteroposterior knee X-rays using the Kellgren-Lawrence (K/L) grading system in those with knee joint symptoms. Knees showing a K/L grade ≥ 2 were classified as knee radiological osteoarthritis (ROA) according to the ACR criteria. In this study 327 subjects underwent knee X-ray tests, and the presence and severity of bony changes for OA were determined twice by experts in rheumatology; (1) no abnormal radiological finding was designated as grade 0; (2) no joint space narrowing but suspicious osteophytes, grade 1; (3) minimal joint space narrowing and definite osteophytes, grade 2; (4) joint space less than 3 mm, severe intra-articular irregularity, and prominent osteophytes, grade 3; (5) no visible joint space and severe sclerosis, grade 4 [29]. The higher K/L grade between both knees was selected. The intraobserver reliability was 0.78 indicating moderate agreement. SMI, PBF, BMR, and BMI were compared according to the K/L grade of knees.
Statistical analyses
Values are expressed as mean ± SD. BIA measurements were compared using independent Student t test, analysis of variance (ANOVA) test, and paired t test. Correlation analyses were performed using Pearson correlation test, cross-tab with chi-square test, linear and logistic regression tests. Four years follow-up BIA values were evaluated using paired t and repeated measured ANOVA test. Cohen’s κ test was used for determination of the intraobserver variation. The results were considered statistically significant if p < 0.05 in 95% confidence interval (CI) and p < 0.001 in 99% CI. Statistical analyses were performed using SPSS version 12 (SPSS Inc., Chicago, IL, USA).
RESULTS
The mean and SD of SMI was 43.53% ± 3.55% in healthy males (n = 2,959) and 37.99% ± 3.19% in healthy females (n = 2,764) aged between 18 and 39 years. According to Janssen’s classification for sarcopenia [26], cases with a SMI greater than 39.98%, between 36.43% and 39.98%, and less than 36.43%, were designated as the normal, class I and class II sarcopenia, respectively, in males; greater than 34.80%, between 31.61% and 34.80%, and less than 31.61% as the normal, class I and class II sarcopenia, respectively, in females based on the reference values (Table 1).

Reference values for the classification of sarcopenia from a sex-matched, healthy Korean population aged between 18 and 39 years
A total of 23,473 subjects, 13,006 males and 10,467 females who underwent BIA were included. The mean age of subjects was 47.92 ± 11.16 and 47.58 ± 11.91 years, respectively. Based on the weight-adjusted SMI, 1,841 males (1,575 class I, 266 class II) and 2,737 females (2,178 class I, 559 class II) were classified according to sarcopenia, demonstrating a higher incidence of sarcopenia in females than in males (Table 2). In the aspect of metabolic rate, BMR showed a steady decrease with the advance of age (p < 0.01) and SMI positively correlated with BMR (r = 0.72, β = 30.96, p < 0.01). The results may indicate that SMI are changing consistently with aging metabolism.
In the purpose of proving the changes of SMI in association with age, BIA was followed up at a 4-year interval in 266 subjects (164 males, 102 females) and evaluated in paired comparisons. During the 4 years the skeletal muscle component showed a significant decrease by –0.33% for SMI (p < 0.01); however, no significant interval change was observed for BMI (p = 0.45). BMR decreases with aging (p < 0.01) consistently with the decreases of SMI. The prevalence was 4.50% (10/222) for sarcopenia during the 4 years follow-up. The results may indicate that SMI is decreasing in the correlation with aging (Table 3).
When the population was divided at 50 of age, an ACR criterion for diagnosis of OA, the population aged over 50 showed a decrease of SMI with the advance of age (r = –0.166, β = –0.112, p < 0.01) in contrast to the population under age 50. The change of SMI in the population over 50 of age was as great as two folds of that in the total population, and 25 to 28 folds of that in the population under 50. Sarcopenia is defined as age-related loss of SMM and a degenerative process of aging. In this study, a negative correlation was observed between SMI and age (r = –0.157, p < 0.01), which was more prominent in females (r = –0.313, p < 0.01) than in males (r = –0.164, p < 0.01). Regression analyses of SMI by years of age showed that the SMI will decrease by 0.061% per year (p < 0.01), and a higher decreasing ratio was observed in females (β = –0.085, p < 0.01) than in males (β = –0.046, p < 0.01) (Table 4).
Logistic regression analyses between SMI and K/L grade adjusted by age, BMI and PBF showed a significantly high frequency of a higher K/L grade (grade 3 and 4) in subjects with a lower SMI (p < 0.01). However, the difference by SMI was not significant in K/L grade 0 and 1 (Table 5). Effects of BMI and BMR on the K/L grade were not consistent or statistically significant.
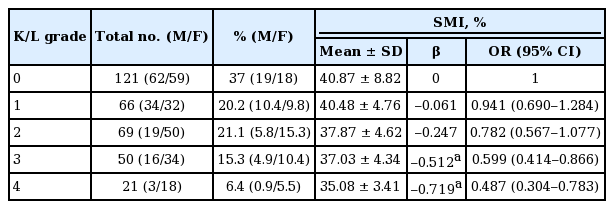
Correlation of SMI and the K/L grade of knee joints with multiple regression analyses for age, body mass index, and PBF
Analyses of BIA determinants in the aspect of bony changes of OA showed that SMI differed statistically among the K/L grades (p < 0.01) contrary to BMI, lean body mass, and SMM. Subjects with the lower SMI tended to be the higher in K/L grade (p < 0.01). In addition, the SMI of subjects with ROA was significantly lower than that of non-ROA, and knees with sarcopenia were prone to be classified as ROA (p < 0.01). As compared with the residuals, the difference between the observed value and the estimated value of the quantity of interest, knees of subjects with normal SMI were prone to be designated as K/L grade 0 or 1, however, knees with sarcopenia showed a significant propensity to be designated as K/L grade 3 or 4 with a trend toward the higher K/L grade in the higher sarcopenia class (p < 0.01) (Table 6).
DISCUSSION
The mean and cut-off values for classification of sarcopenia were relatively higher in Korean people than in those (42.5% ± 5.5% in males, 33.1% ± 5.5% in females) of New Mexico Aging Process Study [24]. Differences between the reference values of SMI may be closely related to differences of ethnicity. It is natural for the SMM to diminish after the adolescent period, and the decrease can be as much as 0.5% to 1% per year after 25 years of age [30]. Loss of SMM, quality, and strength in association with the advance of age refers to sarcopenia as a degenerative process [31]. SMM is active in metabolism, and consistent changes of SMM with aging may be one of the most relevant biomarkers of senescence in the aspect of metabolic alteration [16].
The current study shows a negative correlation between SMI and years of age. BMR decreased with the advance of age, showing a strong positive correlation with SMI. Based on the results, sarcopenia may be the metabolic consequences of aging, and the decreasing SMI can be a reliable indicator of aging.
In the current study, the significance of SMI, age, and BMI was analyzed on age-related OA as compared with the K/L grade of knee joints. Sarcopenia may affect the presence and severity of ROA in association with aging. A higher K/L grade was more frequent in the population with sarcopenia of a lower SMI. When K/L grades 2, 3, and 4 are classified as ROA according to the ACR criteria for OA, subjects accompanying knee ROA had a lower SMI than those with non-ROA, and bony changes of knee joints were found to be more advanced in association with a lower SMI. The probability of a higher K/L grade and the incidence of ROA were increased in the population with a lower SMI; inversely, knees with a higher SMI showed a higher probability of being a lower K/L grade. The results may provide the significance of sarcopenia on development of OA with aging.
Some studies have reported correlation of sarcopenia, sarcopenic obesity and BMI with functional impairment, physical disability, and performance. Lee et al. [32] observed a contribution of BMI-defined sarcopenic obesity to OA suggesting that it is more important than sarcopenia or BMI alone. Scott et al. [33] reported that knee and hip pain may directly affect the progression of sarcopenia in older women, and a higher baseline score of knee ROA may predict a greater increase in the mean lean mass of the leg in both sexes. And Visser et al. [34] reported that a high FM/SMM ratio seems to be unfavorable in knee OA.
In the current study, a time-sequential BIA on the changes of body mass and composition in a paired 266 cases made it clear that it is not BMI but SMI that correspond with aging. Paired comparisons following four years of BIA proved that SMI is decreasing with the advance of age as the normal consequences of senescence. In addition to the cross-sectional correlation between SMI and ROA, the results may be suggestive of a pathogenic correlation between sarcopenia and development of OA with aging. The altered body composition may not be substantial until the end of the fifth decade [35]; however, loss of fat-free mass is augmented and the effects are more prominent in older people. Alterations of growth hormone [36], insulin [37], estrogen, and testosterone [38]; increased levels of interleukin 6 and tumor necrosis factor α [39]; cellular dysfunction and apoptosis with mitochondrial damage [11], and decreased dietary protein intake [13] can be factors contributing to the loss of SMM and strength with aging. After reaching the maximum around 20 years of age, fat-free mass, primarily SMM, will decrease by up to 40 % until 70 years of age [40,41].
This study provides evidence that the presence and severity of ROA are inversely correlated with the age-related loss of SMM. Sarcopenia related with aging may interactively correlate with age-related OA in development and progression. However, because of cross-sectional design of the study, further prospective study is needed to confirm the causal-relationship between sarcopenia and OA development.
KEY MESSAGE
1. Age-related osteoarthritis (OA) is characterized by degradation of articular cartilage and loss of matrix, as a consequence of senescence.
2. The aging process accompanies altered body composition with degenerative loss of skeletal muscle mass and strength, which is referred to as sarcopenia.
3. Sarcopenia related with aging may interactively correlate with the presence and severity of age-related OA.
Notes
No potential conflict of interest relevant to this article was reported.