New monoclonal antibody-based test for Helicobacter pylori urease in gastric tissue
Article information
Abstract
Background/Aims:
To evaluate a new monoclonal antibody for Helicobacter pylori urease in gastric tissue.
Methods:
A total of 107 volunteers were enrolled. All subjects underwent a 13C-urea breath test and esophagogastroduodenoscopy. Gastric aspirates were analyzed for pH and ammonia. Six biopsy specimens in the gastric antrum and body were obtained for a rapid urease test and histology. The new monoclonal antibody-based H. pylori urease test (HPU) was performed to rapidly and qualitatively detect urease in two biopsy specimens.
Results:
H. pylori infection was diagnosed in 73 subjects. The sensitivity and specificity of the HPU was 89% and 74%, respectively. The subjects were divided into two groups: one with true-positive and true-negative HPU results (n = 90) and the other with false-positive and false-negative HPU results (n = 17). Across all subjects, ammonia levels were 900.5 ± 646.7 and 604.3 ± 594.3 μmol/L (p > 0.05), and pH was 3.37 ± 1.64 and 2.82 ± 1.51 (p > 0.05). Sensitivity was higher in the presence of atrophic gastritis or intestinal metaplasia.
Conclusions:
HPU detected H. pylori in approximately 10 min. Gastric aspirate ammonia and pH levels did not affect the test results. Sensitivity was good in the presence of atrophic gastritis or intestinal metaplasia.
INTRODUCTION
Helicobacter pylori is a gram-negative, spiral-shaped bacterium that is estimated to infect more than half of the world’s population, predominantly in developing countries [1]. H. pylori infection is a well-established cause of gastritis, gastric and duodenal ulcers, and duodenitis. H. pylori has been linked to gastric carcinogenesis and mucosa-associated lymphoid tissue lymphoma [2].
Diagnostic methods for detecting H. pylori infection are either invasive, requiring endoscopy to obtain a biopsy, or non-invasive, including serology, a 13C-urea breath test (UBT) [3], and the stool antigen test. Endoscopic tests include histology, the rapid urease test (RUT), and culture-based approaches. These approaches are advantageous for detecting pathological changes, such as a gastric malignancy or ulcer, during the endoscopic examination.
RUT is based on H. pylori urease activity, which splits the urea test reagent to form ammonia. The first RUT (the CLO test) received regulatory approval to be read at 24 hours [4]. The principal disadvantage of this test is that the majority of patients do not have their test results when they leave the endoscopy unit. Thus, a more rapid method is needed.
The aim of this study was to evaluate the efficacy of a new monoclonal antibody-based test to rapidly detect urease in a gastric tissue biopsy specimen. We also attempted to estimate the accuracy of this H. pylori test according to the presence of atrophic gastritis (AG) or intestinal metaplasia (IM).
METHODS
Subjects
In total, 107 subjects were recruited from January 2012 to May 2012 at Saint Carollo Hospital. The subjects were interviewed, and all clinical information was acquired using data collection forms. All subjects gave written informed consent to undergo the esophagogastroduodenoscopy (EGD) and biopsy procedures. Subjects were excluded from the study if they had taken antibiotics, proton pump inhibitors, or bismuth compounds in the previous 2 weeks or had undergone H. pylori treatment. Subjects with renal insufficiency or liver cirrhosis were also excluded. This study protocol was approved by the Ethics Committee at Saint Carollo Hospital.
13C-urea breath test
The UBT used film-coated 13C-urea tablets. Breath specimens were collected 0 and 20 minutes after administration of the UBT tablet, and the δ-13CO2 (UBT value) was measured by infrared spectrometry using a model UbiT-IR300 apparatus (Otsuka Pharmaceutical, Otsuka, Japan). The cut-off value for the UBT was 2.5‰ at 20 minutes. When the UBT value was < 2.5‰ or ≥ 2.5‰, test results were evaluated as negative and positive, respectively.
Endoscopy and biopsy sampling
Subjects underwent EGD after the UBT. After inserting the endoscope into the stomach, gastric juice was aspirated from the fundal pool and discarded. A total of 10 to 20 mL was collected in a trap through the suction channel after 40 mL of distilled water was sprayed in the antrum to rinse the gastric mucosa. Gastric aspirate pH was measured with a Perphect LogRmeter model 370 glass electrode pH meter (Orion, Rockford, IL, USA). Gastric aspirate ammonia was also measured using a Dimension RxL Max device (Siemens, Erfurt, Germany). Six biopsy specimens were taken for histology and RUT from the antrum and body to detect a current H. pylori infection. The new monoclonal antibody-based test was performed with two biopsy specimens acquired from the gastric antrum and body.
Rapid urease test
One antral and one corpus biopsy specimen were used for the RUT (ASAN Helicobacter Test, Asan Pharmaceutical, Seoul, Korea). A color change within 24 hours was regarded as positive.
Histological examination
Two biopsy specimens from the antrum and two from the body were fixed in formalin. The presence of H. pylori was assessed by modified Giemsa staining. The degrees of AG and IM were assessed by hematoxylin and eosin staining. The degrees of AG and IM were assigned to each graded variable (−, absent; +, mild; ++, moderate; +++, severe) [5]. All biopsies were examined by an experienced gastrointestinal pathologist.
H. pylori urease test
The H. pylori urease (HPU) (Ameritek Inc., Everett, WA, USA) is the new monoclonal antibody-based test that utilizes a unique antibody to selectively identify the H. pylori antigen in gastric tissue or saliva. According to the manufacturer, the analytical sensitivity of the test is 5 ng/mL H. pylori urease. Two biopsy specimens were added to a test tube and diluted with eight drops of extraction buffer. The biopsy specimens were swirled vigorously to mix the buffer for about 15 seconds. Four drops of the mixture were added to the sample well of the test kit. As the test reaction commenced, a colored band appeared in the left section of the results window to show that the test was working properly (C). The right section of the results window indicated the test result (T) (Fig. 1). The presence of two color bands within the results window indicated a positive result. The results were interpreted after 5 to 15 minutes.
Definition of H. pylori status
H. pylori infection status was considered positive based on the concordance of at least two of the three tests (UBT, histology, and RUT) [6].
Statistical analysis
Standard methods were used to calculate the sensitivity, specificity, and predictive values of the positive and negative results. Differences in gastric aspirate ammonia and pH were determined using the independent sample t test. A p < 0.05 was considered significant.
RESULTS
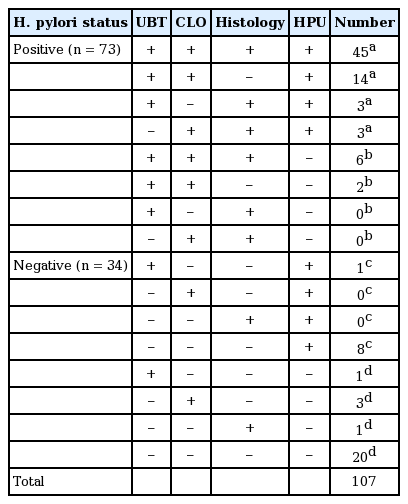
Seventy-three of the 107 enrolled subjects were H. pylori-positive and 34 were H. pylori-negative, according to the H. pylori status definition (Table 1). Age and sex were not significantly different between the H. pylori-positive subjects (male:female, 36:37; mean age, 40.8 ± 9.3 years) and H. pylori-negative subjects (male:female, 15:19; mean age, 40.9 ± 11.3 years). No complications were observed during EGD. Peptic ulcer disease was present in four (5.5%) subjects and one (2.9%) each of the H. pylori-positive and H. pylori-negative subjects, respectively. Gastroesophageal reflux disease was absent in H. pylori-positive subjects but was present in nine H. pylori-negative subjects (26.5%). AG or IM was present in 23 (31.5%) and seven (20.6%) of the H. pylori-positive and H. pylori-negative subjects, respectively. As expected, the prevalence of peptic ulcer disease and AG or IM was relatively high in the H. pylori-positive subjects. AG was evident in 19 cases (17.8%) on the histological examination, whereas IM was observed in 14 cases (13.1%). Of these, three cases had both AG and IM. Among the 30 participants with a histological diagnosis of AG or IM, the degree of AG and IM was graded as mild to moderate (n = 16, 84.2%; n = 10, 71.4%, respectively).
Gastric aspirate ammonia levels were 180 to 2,616 μmol/L in H. pylori-positive subjects and 56 to 659 μmol/L in H. pylori-negative subjects. Gastric aspirate pH was 1.56 to 7.67 in H. pylori-positive subjects and 1.86 to 3.14 in H. pylori-negative subjects. H. pylori-positive subjects had a higher pH (3.66 ± 1.59 vs. 2.37 ± 0.20, p < 0.05) and ammonia level compared to the H. pylori-negative subjects (1,112.5 ± 499.0 vs. 201.2 ± 59.9, p < 0.0001).
Gastric aspirate ammonia levels were 45 to 2,616 μmol/L in the absence of AG or IM and 56 to 1,780 μmol/L in the presence of AG or IM. Gastric aspirate pH was 1.56 to 7.20 in the absence of AG or IM and 1.80 to 7.67 in the presence of AG or IM. Gastric aspirate ammonia and pH were less affected by AG or IM. Ammonia levels in gastric aspirates were 874.5 ± 520.1 and 818.8 ± 704.6 μmol/L in the absence and presence of AG or IM, respectively (p > 0.05). The pH levels in gastric aspirates were 3.87 ± 2.01 and 3.03 ± 1.35 in the absence and presence of AG or IM, respectively (p > 0.05).
Among the 73 H. pylori-positive subjects, 8 were negative on the HPU and 9 of 34 H. pylori-negative subjects were positive on the HPU, based on the H. pylori status definition. The sensitivity and specificity of the HPU was 89% and 74%, respectively. The positive and negative predictive values of the HPU were 88% and 76%, respectively (Table 2).

Sensitivities, specificities, and predictive values for positive and negative monoclonal antibody-based Helicobacter pylori urease test results to detect H. pylori
In 77 subjects without AG or IM, 7 of 50 H. pylori-positive subjects were negative on the HPU and 5 of 27 H. pylori-negative subjects were positive on the HPU. In the 30 subjects with AG or IM, 1 of 23 H. pylori-positive subjects was negative on the HPU and 4 of 9 H. pylori-negative subjects were positive on the HPU. Sensitivity was higher (96%) and specificity was equal (74%) in the presence of AG or IM (Table 2).
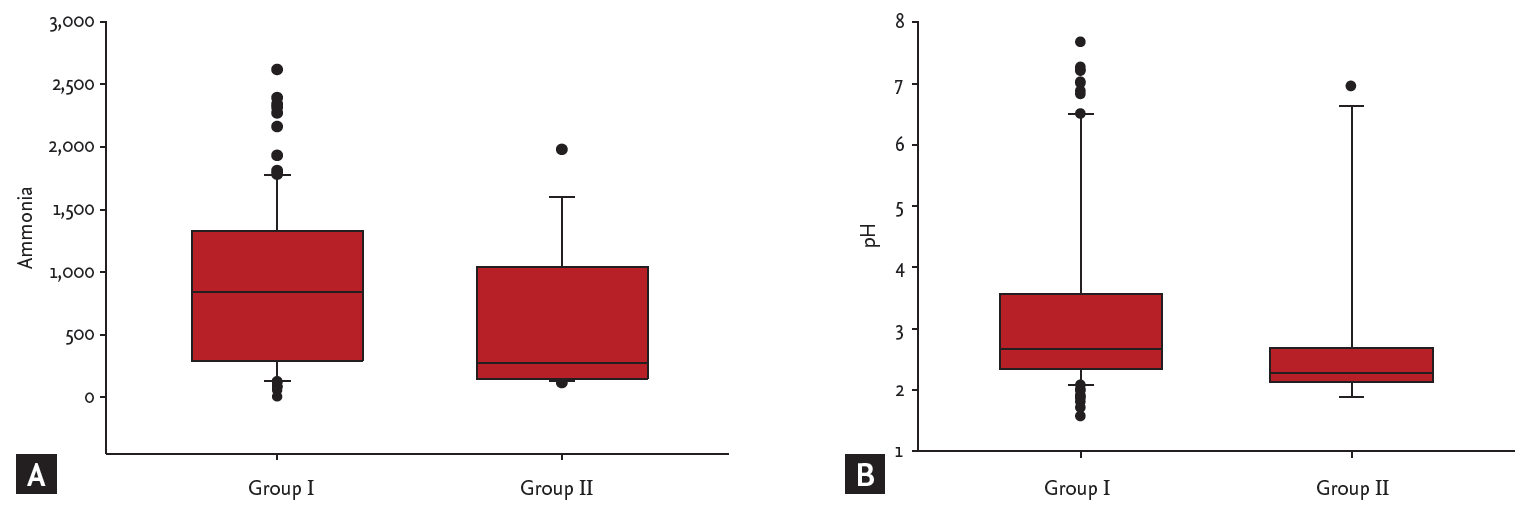
The 107 subjects were divided into two groups according to the H. pylori status definition, i.e., into a group with true-positive and true-negative HPU results (group I, n = 90) and a group with false-positive and false-negative HPU results (group II, n = 17). Ammonia levels in gastric aspirates were 56 to 2,616 μmol/L in group I subjects and 115 to 1,978 μmol/L in group II subjects (p > 0.05). pH in gastric aspirates was 1.56 to 7.67 in group I and 1.89 to 6.95 in group II (p > 0.05) (Fig. 2).
DISCUSSION
Indications for H. pylori eradication are all patients with gastric and duodenal ulcers who are H. pylori positive whether the ulcer is active or in remission, patients with low grade mucosa-associated lymphoid tissue lymphoma, and patients who have undergone resection of early gastric cancer [7]. EGD is required to determine whether patients have a gastroduodenal disease with H. pylori.
RUT has been widely used because it is simple, inexpensive, and easy to carry out [8]. However, a limitation of the urease test is the bacterial load necessary to obtain sufficient sensitivity. Semiquantitative histological evaluations of the bacteria present have clearly shown that false-negative urease test results correspond to the lowest histological scores for H. pylori [9-11]. Another reason for a false-negative result is the presence of IM, which also corresponds to an in-hospital H. pylori environment [12]. Stains used during EGD, such as methylene blue, may blunt the performance of the urease test, so specimens must be taken before employing these stains [13]. The RUT takes 1 to 24 hours to complete. Most endoscopists read RUT results earlier than recommended, which leads to a marked decrease (20%) in sensitivity [14].
Among the noninvasive indirect tests, the UBT has the best sensitivity of approximately 95% [15]. However, false-negative results may also occur when proton pump inhibitors and antibiotics are used. The UBT should be performed separately to detect H. pylori after EGD in patients with gastroduodenal diseases.
The H. pylori stool antigen (HpSA) test has the advantage of being a direct noninvasive test because it detects H. pylori antigens in an easily obtained specimen [16,17]. The monoclonal antibody-based HpSA test has high specificity and sensitivity [18]. However, the lowest concentration was undetectable after only a 24-hour delay in experimentally spiked specimens [19]. Treatment with the mucolytic agent N-acetylcysteine decreases both specificity and sensitivity [20]. While the test is quite specific, it is possible that rare Helicobacter species present in stool may also be detected.
We identified H. pylori urease in gastric tissue specimens using a rapid immunochromatographic assay that utilizes unique antibodies to selectively identify H. pylori antigen in gastric tissue. The test recognized H. pylori urease in the presence of AG or IM. The performance of the kit was unaffected by gastric aspirate ammonia and pH levels. The HPU offers comparable accuracy with a substantially shorter development period to read positive and negative results. In our study, the gold standard for the presence of H. pylori infection status was positivity on at least two tests (UBT, histology, and UBT). If positivity on one of the three tests is indicated by the presence of a H. pylori infection, test performance could differ from our assessment. However, no single test has been considered the gold standard for diagnosing H. pylori until now. As generally recommended, H. pylori infection was considered positive based on the concordance of at least two test results (UBT, histology, and RUT) [6]. A limitation of this study is that the specificity of the HPU was low, and the number of subjects with AG or IM was small.
The HPU is not difficult to perform, and no complications occurred during EGD. The HPU is a useful test to detect H. pylori before patients leave the endoscopy unit. However, further study is needed to use the HPU for clinical applications.
KEY MESSAGE
1. The new monoclonal antibody-based test (Helicobacter pylori urease [HPU]) provided rapid and qualitative detection of HPU.
2. The HPU detected H. pylori urease in the presence of atrophic gastritis or intestinal metaplasia. It was unaffected by gastric aspirate ammonia or pH levels.
Notes
No potential conflict of interest relevant to this article was reported.