Allopurinol hypersensitivity syndrome in patients with hematological malignancies: characteristics and clinical outcomes
Article information
Abstract
Background/Aims
Allopurinol is a urate-lowering agent that is commonly used to prevent chemotherapy-related hyperuricemia. Allopurinol hypersensitivity syndrome (AHS) is a disorder involving multiple organs, which may be accompanied by cutaneous adverse reactions. We identified the characteristics and clinical outcomes of chemotherapy-associated AHS in patients with hematological malignancies.
Methods
This retrospective single-center study included 26 AHS patients (11 with and 15 without hematological malignancies) admitted to Seoul St. Mary's Hospital. AHS was defined using the criteria of Singer and Wallace. Comparisons were made using the Mann-Whitney U test and Fisher exact test as appropriate.
Results
In patients with a hematological malignancy and AHS, statistically significant differences were observed in terms of younger age at onset; shorter duration of exposure; higher starting and maintenance doses of allopurinol; lower incidence of eosinophilia, leukocytosis, and underlying renal insufficiency; and more frequent occurrence of fever compared to AHS patients without a hematological malignancy. Two AHS patients with a hematological malignancy were examined for human leukocyte antigen (HLA)-B typing, but neither patient harbored the HLA-B*5801 allele. All of the patients ceased allopurinol treatment, with most patients making a full recovery. Two patients in the study died; however, these deaths were unrelated to AHS. One patient developed serious sequelae of AHS that required hemodialysis.
Conclusions
Physicians who prescribe allopurinol for the prevention of chemotherapy-related hyperuricemia should be aware of the unique risk of AHS, even in patients with hematological malignancies who do not have known risk factors for AHS. Novel urate-lowering agents should be considered alternative treatments.
INTRODUCTION
Allopurinol is a xanthine oxidase inhibitor that is widely used to treat recurrent acute gouty arthritis, tophi, urate nephropathy, and uric acid kidney stones [1], and can be used prophylactically to prevent chemotherapy-induced hyperuricemia. Use of allopurinol for the treatment of asymptomatic hyperuricemia has also become common, due to a series of reports demonstrating improvements in chronic kidney disease (CKD) and improved cardiovascular outcome in high-risk patients following allopurinol treatment [23].
Allopurinol hypersensitivity syndrome (AHS) was first reported by Lang [4] in 1979, and diagnostic criteria were established in 1986 [5]. AHS is categorized as a severe cutaneous adverse reaction (SCAR) [6]. Recent EuroSCAR studies demonstrated that allopurinol is the leading cause of Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) in Europe and Israel [78]. Furthermore, mortality associated with AHS has been estimated at 32%, which is higher than other drug-induced SCARs [7].
Allopurinol has long been used for the prevention of hyperuricemia in patients undergoing chemotherapy, particularly leukemia and lymphoma patients who are more susceptible to developing tumor lysis syndrome [910]. The majority of current treatment guidelines, including those set forth by the National Comprehensive Cancer Network, recommend allopurinol as first-line therapy for patients with hyperuricemia accompanied by tumor lysis syndrome [1112]. Hematological malignancies are often accompanied by severe comorbidities such as infection and neutropenia. Developing AHS can significantly increase the duration of treatment when accompanied by these aforementioned comorbidities.
In the present study, we identified differences between cases of AHS in patients with and without a hematological malignancy. We also investigated the clinical characteristics and treatment outcomes for AHS in patients with hematological malignancies.
METHODS
Patients and controls
We retrospectively reviewed the medical records of patients with AHS who were treated at Seoul St. Mary's Hospital, Korea, a tertiary care university hospital and referral center, between January 2005 and June 2013. All of the study protocols were approved by the Institutional Review Board of Seoul St. Mary's Hospital prior to initiation of the study.
Allopurinol was prescribed to prevent chemotherapy-related hyperuricemia in all patients with hematological malignancies. Of the 3,227 patients admitted for treatment of a hematological malignancy, 11 developed AHS. The control group consisted of 2,785 patients receiving allopurinol for the treatment of recurrent gouty arthritis, tophi, urate nephropathy, uric acid kidney stone, and asymptomatic hyperuricemia. From this cohort, 15 patients were identified as having AHS that was not accompanied by a hematological malignancy. Subjects were excluded from this analysis if they had incomplete clinical data or a definitive hypersensitivity to another drug. The final cohort of AHS patients consisted of 11 patients with, and 15 patients without, a hematological malignancy; all of the patients were Korean.
Definitions and variables
AHS was defined using the standard criteria set forth by Singer and Wallace [5]. These criteria consist of a clear history of exposure to allopurinol, and a lack of exposure to another drug known to cause similar clinical symptoms, along with two of the major criteria (worsened renal function, acute hepatocellular injury, or rash) or one major criterion and one minor criterion (fever, eosinophilia, or leukocytosis). The definition of rash included SJS, TEN, erythema multiforme (EM), diffuse maculopapular rash, or exfoliative dermatitis. Severe skin manifestation was defined as SJS or TEN. Renal function measurements were based on serum creatinine levels and the estimated glomerular filtration rate (eGFR) calculated using the Modification of Diet in Renal Disease equation. Pre-existing renal impairment was defined as baseline eGFR < 60 mL/min. Acute hepatocellular injury was defined as aspartate aminotransferase > 35 IU/L or alanine aminotransferase > 35 IU/L. Leukocytosis and eosinophilia were defined as white blood cell (WBC) counts > 11.0 × 106 cells/L and eosinophil counts > 6% of the total WBC count [13].
Demographic, clinical, and laboratory profiles
Along with the standard demographic characteristics (age, sex, and underlying disorders), clinical, laboratory, and HLA allele data were collected at the time of AHS onset. Information about the presence of major and minor criteria for AHS, the starting and maintenance dosages of allopurinol, duration of exposure to allopurinol, concomitant use of diuretics, AHS treatment modalities, and clinical outcomes of AHS were also obtained.
Statistical analysis
Comparisons of continuous values between the two groups were performed using the Mann-Whitney U test. Results are presented as median with interquartile range. Categorical variables, such as proportions, were compared between groups using the Fisher exact test. Values of p < 0.05 were indicated statistical significance. All of the tests were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Clinical characteristics and laboratory profiles of 11 AHS patients with hematological malignancies
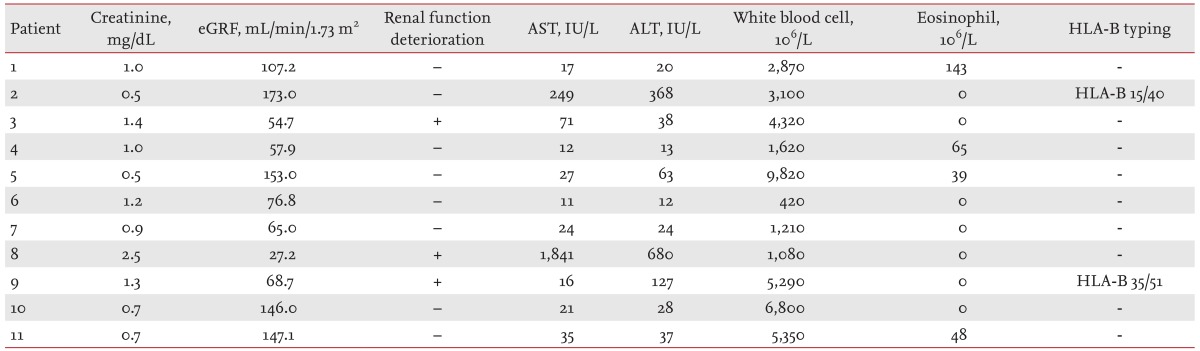
Clinical characteristics of the 11 AHS patients with hematological malignancies are summarized in Table 1. Seven patients had underlying lymphoma, three had acute myeloid leukemia, and one had essential thrombocytosis. With the exception of patient 3, all of the patients exhibited skin manifestations of AHS. Of the 10 patients presenting with skin manifestations, only patient 8 had severe skin manifestations; 10 of the 11 patients presented with an accompanying fever. The duration of allopurinol exposure ranged from 5 to 23 days. Starting doses ranged from 300 to 450 mg, with a standard 300 mg maintenance dose used in all AHS patients with hematological malignancy.
Laboratory results for the 11 AHS patients with hematological malignancy are shown in Table 2. Three patients exhibited deterioration of renal function, and five had elevated transaminase levels at the time of AHS onset. None of the patients displayed leukocytosis or eosinophilia. Two of the patients were screened for human leukocyte antigen (HLA) type, although neither patient was found to carry the HLA-B*5801 allele.
Comparisons between AHS patients with and without a hematological malignancy
Next, clinical and laboratory characteristics were compared between AHS patients with and without a hematological malignancy (Table 3). Statistically significant differences were observed in onset age, duration of exposure to allopurinol, starting and maintenance dosages of allopurinol, percentage of combined leukocytosis and eosinophilia, presence of fever as a manifestation of AHS, and existence of underlying renal impairment. The median age of AHS onset was younger in patients with a hematological malignancy (39.0 years [31.0 to 64.0] vs. 60.0 years [50.0 to 72.0], p = 0.047). The development of AHS was more rapid in patients with a hematological malignancy than those without a hematological malignancy (10.0 days [7.0 to 12.0] vs. 35.5 days [16.8 to 70.0], p = 0.003). Both the starting and maintenance doses of allopurinol were significantly higher in AHS patients with a hematological malignancy (starting dose, 300.0 mg vs. 100.0 mg [100.0 to 200.0], p < 0.0001; maintenance dose, 300.0 mg vs. 100.0 mg [100.0 to 200.0], p < 0.0001). None of the AHS patients with a hematological malignancy exhibited leukocytosis, whereas eight of the AHS patients without a hematological malignancy displayed leukocytosis (0% vs. 53.3%, p = 0.007). There was a significant difference in the frequency of eosinophilia in AHS patients with and without a hematological malignancy (0% vs. 40%, p = 0.024). In total, 10 of the 11 AHS patients with a hematological malignancy presented with fever, compared to only seven of 15 AHS patients without a hematological malignancy (90.9% vs. 46.7%, p = 0.036). Pre-existing renal impairment was significantly higher in AHS patients without a hematological malignancy (0% vs. 66.7%, p = 0.001). The frequencies of concomitant use of diuretics and accompanying severe skin manifestations were lower in AHS patients with a hematological malignancy; however, these differences did not reach statistical significance.
Treatments and clinical outcomes of 11 AHS patients with a hematological malignancy
The medications and clinical outcomes of the 11 AHS patients with a hematological malignancy are shown in Table 4. All of the 11 patients stopped receiving allopurinol. Eight were treated with a systemic corticosteroid, six with a topical corticosteroid, nine with an applied antihistamine, and three with applied hepatotonics. A combination of systemic and topical corticosteroids was prescribed for four of the AHS patients with a hematological malignancy. Two of these patients eventually died; one as a result of a secondary pneumonia and the other from a brain hemorrhage. The patient who died as a result of a brain hemorrhage also had an acute kidney injury requiring hemodialysis that was attributed to AHS.
DISCUSSION
Allopurinol has been used since the 1960s to prevent recurrent acute gouty arthritis [14]. It is a structural isomer of hypoxanthine that is capable of inhibiting xanthine oxidase, resulting in decreased serum urate levels. Indications for allopurinol include recurrent acute gouty arthritis, urate-induced nephropathy or lithiasis, tophi formation, and tumor-lysis-syndrome-related hyperuricemia. Allopurinol is not currently indicated for the treatment of asymptomatic hyperuricemia, but many physicians prescribe it for asymptomatic hyperuricemia based on the results of a series of recent studies. Hyperuricemia is an independent risk factor for several diseases, including hypertension, cardiovascular disease, renal disease, and metabolic syndrome [151617]. Limited data demonstrating a protective effect of allopurinol in cardiovascular disease and CKD have led to the increased use of allopurinol in these patients [23], with a proportional increase in the allopurinol-induced conditions SJS and TEN [78]. Considering that allopurinol is primarily used as first-line therapy for chemotherapy-related hyperuricemia [1112], AHS has emerged as an important health-care-related complication.
Since a case in which allopurinol caused a severe cutaneous complication with systemic symptoms was first described by Kantor [18], AHS has been occasionally reported. Singer and Wallace [5] suggested diagnostic criteria for AHS in 1986; since then, several retrospective studies have been performed to identify risk factors for AHS. In a meta-analysis of all published cases of AHS to date, Ramasamy et al. [19] identified several risk factors for AHS, including pre-existing renal insufficiency, concomitant use of diuretics, recent initiation of allopurinol, and presence of the HLA-B*5801 allele. Of these, only the HLA-B*5801 allele was identified as a definite risk factor for AHS, whereas those remaining are suspected risk factors [19]. The HLA-B*5801 genotype has shown increased odds ratios for AHS in multiple studies, particularly among Asian populations [20212223]. Higher odds ratios between HLA-B*5801 and AHS have also been observed in Koreans with CKD and in Han Chinese and Taiwanese populations, compared to European and Japanese cohorts [202122242526]. This suggests that genetic variation other than HLA-B*5801 may have a role in the occurrence of AHS. All of the patients in this study were Korean; two patients who were screened for the HLA-B type did not harbor the HLA-B*5801 allele. Because many hematological malignancies arise from complex genetic backgrounds, such as single-nucleotide polymorphisms and copy number abnormalities [252728], it is likely that genetic variation other than HLA-B*5801 play a role in the development of AHS in patients with hematological malignancies.
In the present study, AHS patients with a hematological malignancy displayed significant differences in a range of clinical covariates compared to AHS patients without a hematological malignancy. A difference in ~21 years was seen in the age of onset between AHS patients with and without a hematological malignancy (p = 0.047). This finding is consistent with other reports of an average age of onset for AHS without a hematological malignancy of ~60 years of age. Indeed, several studies have suggested that old age is a risk factor for AHS [192930]. Therefore physicians have to beware of AHS when administering allopurinol to patients with hematological malignancy even they are younger than usual AHS onset age. The starting and maintenance doses of allopurinol were significantly higher in cases of AHS with a hematological malignancy. Stamp et al. [30] claimed that a starting dose of allopurinol of 1.5 mg per unit eGFR could reduce the risk of developing AHS. Our data indicated no significant difference in starting dose per unit eGFR between AHS patients with and without a hematological malignancy (p = 0.478); however, the average starting dose was significantly higher in AHS patients with a hematological malignancy (p < 0.001). This apparent discrepancy may be due to the higher frequency of underlying renal insufficiency in AHS patients without a hematological malignancy, resulting in lower starting doses and accompanying lower mean eGFR in these patients. New guidelines governing the starting dose of allopurinol have recently been established by the American College of Rheumatology (ACR), with serial dose escalation recommended in tolerant patients [1]. In our study, all of the patients received a higher starting dose of allopurinol per eGFR than recommended by current guidelines, regardless of hematological malignancy status.
Pre-existing renal insufficiency was significantly lower in AHS patients with a hematological malignancy. Despite this being a well-known risk factor for AHS [19], none of the 11 AHS patients with a hematological malignancy exhibited renal impairment prior to the onset of AHS. This observation is important from a clinical standpoint, as it suggests that the absence of renal insufficiency does not indicate a reduced probability of AHS in patients with a hematological malignancy.
The absence of leukocytosis, eosinophilia, and high frequency of fever in AHS patients with a hematological malignancy can be attributed to the influence of chemotherapy-induced neutropenia [31]. All cases of AHS with a hematological malignancy occurred within 23 days following initiation of allopurinol and chemotherapy, with a median duration of 10 days. The onset of AHS was significantly shorter in patients with a hematological malignancy than patients without a hematological malignancy (p = 0.003). Rapid onset of AHS in hematological malignancy patients is meaningful when considered alongside a previous meta-analysis, which reported a mean duration of 10 weeks to AHS onset, substantially longer than that of the AHS patients with a hematological malignancy described here [19].
Finally, although the frequency of concomitant diuretic use was not significantly different between AHS patients with and without a hematological malignancy, patients with a hematological malignancy exhibited a lower overall frequency. In total, seven of the 15 AHS patients without a hematological malignancy (46.7%) used concomitant diuretics, a similar proportion found in a meta-analysis that reported a 45.2% rate of use [19]. This lower percentage of concomitant diuretics use is meaningful, as it indicates that patients with hematological malignancies may develop AHS despite the absence of concomitant diuretic use, which is a suspected risk factor. Because diuretics are usually used in patients with CKD to maintain urine output, the lower frequency of concomitant diuretic use in AHS patients with a hematological malignancy may be affected by baseline characteristics showing a higher frequency of normal renal function.
The incidence of AHS in patients with a hematological malignancy was 0.34% in this study. Side effects of allopurinol are reported in 1% to 5% of patients when mild side effects, such as gastrointestinal intolerance or mild fever, are included [2932]. Recently, Kim et al. [33] reported an incidence of allopurinol-related SCARs of 0.69 per 1,000 person-years. A recent meta-analysis of AHS patients revealed a total mortality rate of 13.8%; the mortality directly attributable to AHS comprised 86.2%. In our study, the overall mortality rate was 18.2%, with all deaths unrelated to AHS. A previous meta-analysis showed that severe cutaneous manifestations were associated with a significantly higher likelihood of mortality due to all causes, including AHS-related mortality [19]. A major cause of death in severe skin manifestations (SJS and TEN) is secondary bacterial infection due to the impaired skin barrier. The patients in our study showed relatively low incidences of SJS and TEN; however, the mortality rate was similar to previous studies. This suggests that even when not accompanied by severe skin manifestations, the risk of death in AHS patients with a hematological malignancy is a concern.
ACR guidelines for the treatment of gouty arthritis recommend starting allopurinol according to the degree of renal impairment, followed by a gradual increase in dosage to reduce the risk of AHS [1]. However, it is not possible to gradually escalate the starting dose of allopurinol according to a patient's tolerance, or adopt a desensitization protocol, in patients undergoing chemotherapy because rapid elevation of urate levels is expected in hematological malignancies [34]. Another option for these patients may exist in the form of febuxostat and rasburicase. Febuxostat is a non-purine selective inhibitor of xanthine oxidase, which is more effective than allopurinol in terms of lowering serum urate concentrations [35]. Furthermore, febuxostat appears to induce hypersensitivity syndrome less frequently than allopurinol, and is well tolerated by most AHS patients [3436]. Rasburicase is a potent recombinant form of urate oxidase, which can metabolize uric acid to the more highly soluble compound allantoin. It has better effects than allopurinol in reducing uric acid levels when used as a preventive drug for tumor lysis syndrome [37]. Therefore, febuxostat and rasburicase may be good alternatives for the treatment of hyperuricemia, particularly in patients with hematological malignancies who are likely to undergo chemotherapy.
Treatment modalities for AHS patients with hematological malignancies are similar to those for AHS patients without hematological malignancies [1938]. The majority of patients recovered without sequelae, with only patient eight experiencing severe sequelae requiring hemodialysis. The incidence of AHS requiring hemodialysis was similar to that of a previous meta-analysis, which reported hemodialysis in 6.6% of patients. The major treatment modality was glucocorticoids; other treatment modalities were used to control concomitant symptoms, such as pruritus. Although intravenous immunoglobulin (IVIg) did not lower the mortality rate in AHS patients [39], IVIg may be beneficial to AHS patients with hematological malignancies, as they are more prone to infection [40].
The present study was not without its limitations. First, comparisons were only performed between AHS patients with and without a hematological malignancy. To clarify the risk factors for AHS in patients with a hematological malignancy, a comparison with patients with hematological malignancies who did not experience AHS following administration of allopurinol should be performed. Second, the presence of genotype HLA-B*5801 was only determined in two AHS patients with a hematological malignancy. This limitation prevented clarification of the association between HLA-B*5801 and AHS in patients with a hematological malignancy. Third, relatively few patients were evaluated. Due to the low incidence of AHS in patients with hematological malignancies, a multicenter study is necessary to overcome this limitation.
To summarize, AHS occurs in patients with hematological malignancies in the absence of most risk factors, including pre-existing renal impairment or the presence of the HLA-B*5801 allele. Physicians who prescribe allopurinol for the prevention of chemotherapy-related hyperuricemia must be mindful of the possibility of development of AHS in this population, even in the absence of common risk factors. Novel urate-lowering agents should be considered alternative agents for the prevention of chemotherapy-related hyperuricemia in this patient population.
KEY MESSAGE
1. Allopurinol hypersensitivity syndrome (AHS) can occur in patients with hematological malignancies who do not otherwise exhibit risk factors for AHS.
2. Physicians must therefore be aware of AHS when prescribing allopurinol to such patients.
Acknowledgments
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI13C0016).
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.