Association of nasal inflammation and lower airway responsiveness in schoolchildren based on an epidemiological survey
Article information
Abstract
Background/Aims
We sought to increase our understanding of the rhinitis-asthma relationship and improve strategies for the treatment of patients with these diseases. The aim of this study was to identify a connection between upper airway inflammation and lower airway responsiveness.
Methods
We counted eosinophils on nasal smears, and performed spirometry, allergic skin tests, and methacholine challenge tests in 308 schoolchildren plus a questionnaire on respiratory symptoms. The methacholine concentration causing a 20% fall in forced expiratory volume in 1 second (PC20 < 25 mg/mL) was used as the threshold of bronchial hyperresponsiveness (BHR).
Results
In total, 26% of subjects had positive nasal eosinophils on a smear, and 46.2% of subjects had BHR at < 25 mg/mL methacholine PC20. Nasal symptoms were higher in subjects with than without nasal eosinophils (p = 0.012). Asthma symptoms did not differ between subjects with and without nasal eosinophils. Nasal eosinophils were higher in subjects with atopy than those without (p = 0.006), and there was no difference in PC20 methacholine according to atopy (15.5 ± 1.07 vs. 17.5 ± 0.62; p > 0.05). No difference in BHR was detected when comparing subjects with and without nasal eosinophils. There were significant differences in the PC20 between subjects with greater than 50% nasal eosinophils and without nasal eosinophils (11.01 ± 2.92 mg/mL vs. 17.38 ± 0.61 mg/mL; p < 0.001).
Conclusions
These findings demonstrated that nasal eosinophilic inflammation might contribute to lower airway responsiveness in schoolchildren, based on an epidemiological survey.
INTRODUCTION
Allergic asthma and rhinitis are manifestations of atopic syndrome and often coexist. It has been demonstrated that allergic rhinitis is a strong risk factor for the onset of asthma in adults [1]. Rhinitis and asthma typically occur together, and there is increasing evidence that allergic rhinitis (AR) influences the clinical course of asthma. The prevalence of AR and asthma varies globally, with AR generally twice as common as asthma [1]. Rhinitis is present in more than 80% of patients with allergic asthma. Moreover, 76% of adult patients with AR and asthma reported rhinitis before the onset of asthma [2]. AR patients without symptoms of asthma often have bronchial hyperresponsiveness (BHR) to non-specific bronchoconstrictors, such as methacholine or histamine [3,4,5,6]. Methacholine responsiveness within the asthmatic range in patients with rhinitis is associated with variable airflow obstruction and subclinical asthma [7,8].
Several mechanisms have been proposed for the interaction between the upper and lower airways in AR and asthma [3,9]. The direct effects are the naso-bronchial reflex, postnasal drip due to inflammatory cells, and/or mediators from the nose into the lower airways, and absorption of inflammatory cells and/or mediators from the nose into systemic circulation and, ultimately, the lungs [5,9]. Indirect effects include nasal obstruction, causing reduced filtration, humidification, and warming by the nose [5,9]. AR and asthma are characterized by a similar inflammatory pattern in which eosinophils and T lymphocytes are the predominant cell types [1]. Eosinophilic inflammation may be present in subjects with AR and BHR, even in the absence of symptoms of asthma [10].
To explore the association between upper airway inflammation and lower airway responsiveness, we examined the results of methacholine challenge tests and nasal eosinophils on nasal smears in schoolchildren using an epidemiological survey.
METHODS
Study population and questionnaire
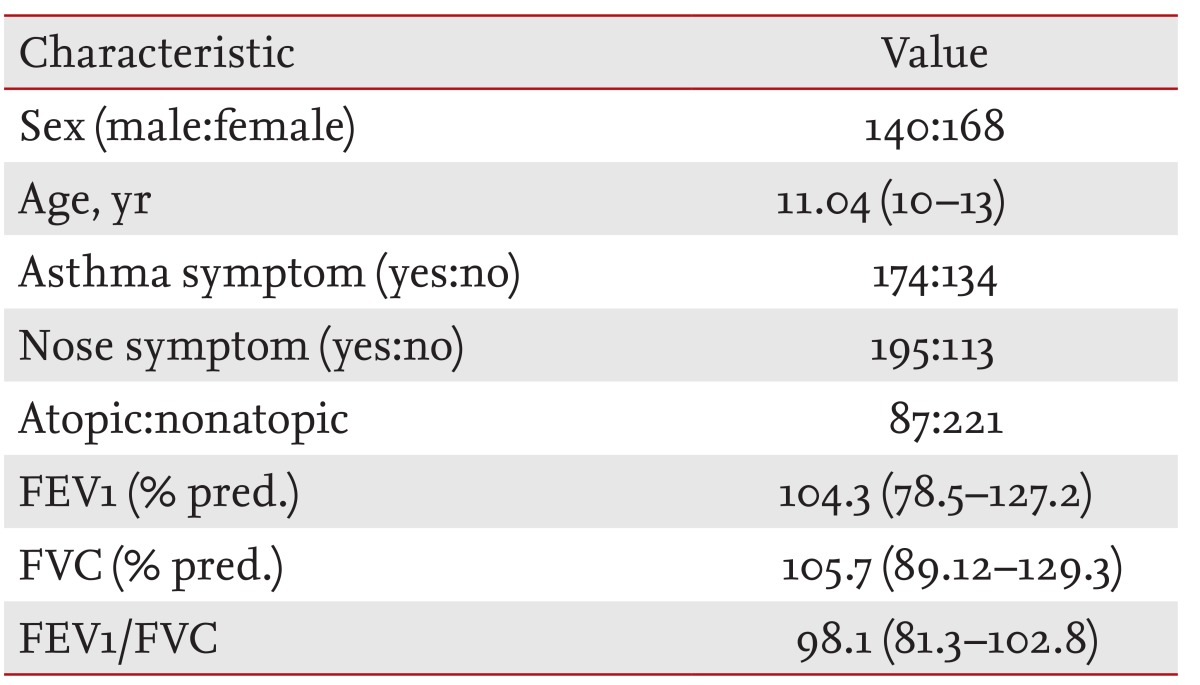
All children were 10 to 13 years of age. A questionnaire on respiratory and allergic disorders was administered including questions developed for the International Study of Asthma and Allergies in Childhood (ISAAC). The focus of this survey was on responses to the ISAAC questions on "wheezing in the last 12 months," "number of wheezing attacks in the last 12 months," and on "sleep-disturbing" and "speech-limiting" wheezing in the last 12 months. Symptoms of AR (sneezing or runny/blocked nose without a cold and itchy-watery eyes) were assessed [11]. Additionally, a question on morning coughing as a symptom of non-specific airway irritation was asked ("Did you frequently cough in the morning right after waking up in the last 12 months?"). In total, 308 schoolchildren were enrolled in the study (Table 1). None of the subjects took any drugs, such as anti-histamines, cromolyn, theophylline, or sympathomimetics, which could interfere with the performance of the skin tests within 72 hours of the tests.
The Ethics Committee of Soonchunhyang University Bucheon Hospital approved the study protocol. All parents/guardians signed informed consent forms before the study.
Nasal swabs for eosinophils
Nasal mucosal specimens were obtained, processed, and cells were counted as described previously, with minor modifications [12]. Briefly, mucosal specimens were scraped from the surfaces of the middle thirds of the inferior turbinates, and were then transferred onto glass slides, fixed in 95% ethyl alcohol, and stained with modified Wright-Giemsa stain. Nasal eosinophils were counted at ×1,000 magnification under a light microscope. At least 10 well-spread, high power epithelium fields were examined independently by two allergists, one of whom was blinded to the clinical status of the patients. The quantitative score of nasal eosinophils was rated according to a scale previously described by a modified Meltzer method [12]. The degree of eosinophil inflammation was evaluated on a subjective scale of 0 to 4. A value of 0 was assigned when no eosinophils were detected, a value of 1 for less than 10% eosinophils, a value of 2 for 10% to 25% eosinophils, a value of 3 for 25% to 50% eosinophils, and a value of 4 for greater than 50% eosinophils.
Spirometry
Spirometry was performed according to the American Thoracic Society standards [13] using a SensorMedics 2200 spirometer (Cardiopulmonary Care Co., Yorba Linda, CA, USA). Representative values for forced vital capacity and forced expiratory volume in 1 second (FEV1) were selected according to the International Thoracic Society criteria [14] and reference values were taken from the report by Choi et al. [15].
Bronchial hyperresponsiveness
Methacholine challenge tests were performed using a modification of the method described by Chai et al. [16]. Concentrations of 0.075, 0.15, 0.31, 0.62, 1.25, 2.5, 5, 10, and 25 mg/mL methacholine were prepared by dilution with buffered saline. A micro-dosimeter (S&M Instrument Co., Doylestown, PA, USA) was used to deliver the aerosol generated by a DeVilbiss 646 nebulizer. Subjects inhaled five breaths of increasing concentrations of methacholine until the FEV1 decreased by more than 20% of its basal value or the highest concentration was reached. The largest value of triplicate FEV1 measurements at 30, 90, or 180 seconds after each inhalation was used for analysis. If PC20 was less than 25 mg/mL, a subject was considered to have BHR to methacholine.
Allergy skin prick tests
Allergy skin prick tests with commercially available inhalant allergens (Dermatophagoides pteronyssinus, Dermatophagoides farinae, Aspergillus spp., alder, birch, hazel, rye, timothy, mugwort, ragweed, and cockroach; Allergopharma Co., Reinbek, Germany) were performed on the volar side of both forearms. None of the subjects had received antihistamines orally for 3 days before the study. A positive control of histamine (1 mg/mL) as well as a negative diluent control was included in all tests. After 15 minutes, the mean diameter of the wheal formed by the allergen was compared with that formed by histamine. A wheal and erythema size equal to or greater than that of histamine (positive control) was read as '3+.' Reactors were defined as exhibiting atopy when they showed a response > 3+ to one or more allergens in the skin prick tests [17].
Statistical analysis
Data were double-entered into a SPSS version 14.0 (SPSS Inc., Chicago, IL, USA) and are expressed as means ± standard deviation or standard error of the mean. Group differences in atopy were compared using a two-sample t test, the Mann-Whitney U test, or Pearson chi-square test for normally distributed, skewed, or categorical data, respectively. Differences in the proportions of the patient populations were analyzed with the chi-square test with Fisher exact test when low cell counts were encountered. A p < 0.05 was considered to indicate statistical significance.
RESULTS
The characteristics of the 308 subjects enrolled are provided in Table 1. In total, 26.2% (81/308) of the subjects had positive nasal eosinophils on smears. Of the subjects, 28.8% (89/308) had atopy on allergic skin tests. Grade 0 nasal eosinophils accounted for 75% (231/308) of the subjects, grade 1 for 11.0% (34/308), grade 2 for 5.2% (16/308), grade 3 for 5.2% (16/308), and grade 4 for 4.2% (13/308).
In total, 46.1% (142/308) of the schoolchildren were BHR-positive, considering < 25 mg/mL of methacholine PC20 as the threshold. Nasal symptoms were higher in subjects with nasal eosinophils than in subjects without (60/195 vs. 21/113, p = 0.012) (Fig. 1). Asthma symptoms did not differ between subjects with and without nasal eosinophils (44/174 vs. 37/134, p > 0.05). Nasal eosinophils were higher in subjects with atopy than in subjects without atopy (38.3% [33/87] vs. 21.1% [47/221], p = 0.006) (Fig. 2). There was no difference in PC20 methacholine according to atopy (15.5 ± 1.07 vs. 17.5 ± 0.62, p > 0.05). No difference in PC20 methacholine was detected when comparing subjects with and without nasal eosinophils. There was no correlation between nasal eosinophils and PC20 methacholine. There was a significant difference in the PC20 between subjects with nasal eosinophils greater than 50% and those without nasal eosinophils (11.01 ± 2.92 mg/mL vs. 17.38 ± 0.61 mg/mL, p < 0.001) (Fig. 3).
DISCUSSION
The present study showed increased nasal eosinophils in subjects with airway hyperresponsiveness, suggesting a link between the upper and lower respiratory tracts. AR and asthma are considered aspects of global airway allergy syndrome [17]. The impairment of purification, humidification, and warming of inspired air by the nose in rhinosinusitis may be partly responsible for the bronchial pathology [18]. Asthma and rhinitis can be associated with both an immunoglobulin E-mediated allergic reaction and an inflammatory pattern. In total, 28% to 50% of asthmatic patients have AR, compared with 10% to 20% of the general population [7]. Many patients with AR (and no perceived asthma symptoms) have BHR to natural stimuli, such as exercise or bronchial challenge with chemical stimuli, such as histamine and methacholine, especially during AR exacerbation [7]. Simons [19] suggested that a new term "allergic rhinobronchitis" accurately described the chronic allergic inflammation in the airways of patients with concurrent AR and asthma. The key to management of both disorders lies in addressing the common immunopathological mechanisms and preventing/relieving chronic allergic inflammation using both appropriate pharmacological treatments (including specific immunotherapy) and by recommending allergen avoidance in selected patients [19]. In this study, nasal symptoms (but not lung symptoms) were higher in subjects with nasal eosinophils than subjects without, suggesting that nasal eosinophilic inflammation contributes to the nasal symptoms.
Several cross-sectional studies [20,21,22,23] have reported a frequent association between AR and asthma. Among them, a European multicenter cross-sectional survey evaluated respiratory symptoms in a young adult population [20], in which ~60% of asthmatic patients had AR. Alternatively, patients with AR presented an 8-fold risk of having asthma versus patients without AR [20]. The severe nasal symptoms were associated with bronchial symptoms in patients with persistent AR [21].
BHR is known to be an important risk factor for asthma development in patients with both perennial AR and seasonal AR [22]. BHR affected 82.2% of patients with perennial AR, 73.6% of patients with mixed AR, and 53.5% of patients with seasonal AR of 2,347 patients with perennial AR, seasonal AR, and mixed AR [23]. In this study, although differences in BHR were not detected when comparing subjects with and without nasal eosinophils, there were significant differences in the PC20 of subjects with nasal eosinophils greater than 50% versus without nasal eosinophils, suggesting that severe nasal inflammation may be a risk factor for asthma.
The immune effector cells responsible for allergic reactions in both the lung and the nose include mast cells, T lymphocytes, and eosinophils [24,25,26]. Eosinophils are characteristic of the acute and chronic inflammatory changes observed in bronchial asthma and AR, and have been implicated in many aspects of tissue damage that occurs at sites of chronic inflammation. The number of eosinophils infiltrating the nasal mucosa correlates well with the most important clinical, functional (e.g., nasal airflow), and immunological parameters, and is significantly associated with nasal eosinophil counts and spirometric findings, including forced expiratory flow from 25% to 75% of vital capacity (FEF25-75), FEV1, and BHR [27,28]. With more intense nasal inflammation, bronchial involvement becomes clearer [27]. Nasal airflow and FEV1 are also significantly correlated with nasal eosinophil counts, and nasal airflow is significantly correlated with FEV1 [29]. There is also a relationship between AR and allergic parameters, such as nasal symptoms and eosinophils [30]. Upper airway inflammatory processes occurring totally or primarily in the upper airway may participate in the pathogenesis of BHR and asthma [22,31].
In agreement with previous studies [22,27,28,29,30,31], an increased number of eosinophils in the nose increased airway hyperresponsiveness in the lungs.
In conclusion, nasal symptoms in children were higher in subjects with nasal eosinophils than those without, suggesting that the nasal symptoms may be due to eosinophilic inflammation. The presence of BHR correlated with an increase in the proportion of eosinophils, suggesting that eosinophils contribute to BHR in AR. Overall, this study showed a relationship between nasal eosinophils and BHR, suggesting that an association between the upper and lower airways may exist in schoolchildren, based on epidemiological surveys.
KEY MESSAGE
Rhinitis symptoms and asthma symptoms can occur together and rhinitis symptoms may influence asthma symptoms.
Nasal eosinophilic inflammation may cause bronchial hyperresponsiveness.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (2013R1A1A2005465), and Soonchunhyang University Research Fund.
Notes
No potential conflict of interest relevant to this article was reported.