The association between an abnormal post-voiding urine volume and a lower estimated glomerular filtration rate in patients with type 2 diabetes with no voiding symptoms
Article information
Abstract
Background/Aims
Diabetic cystopathy is a frequent complication of diabetes mellitus. This study assessed the association between the post-voiding residual (PVR) urine volume and diabetic nephropathy in type 2 diabetics with no voiding symptoms.
Methods
This study investigated 42 patients with type 2 diabetes who were followed regularly at our outpatient clinic between July 1, 2008 and June 30, 2009. No patient had voiding problems or International Prostate Symptom Scores (IPSSs) ≥ 12. An urologist performed the urological evaluations and the PVR was measured using a bladder scan. A PVR > 50 mL on two consecutive voids was considered abnormal, which was the primary study outcome.
Results
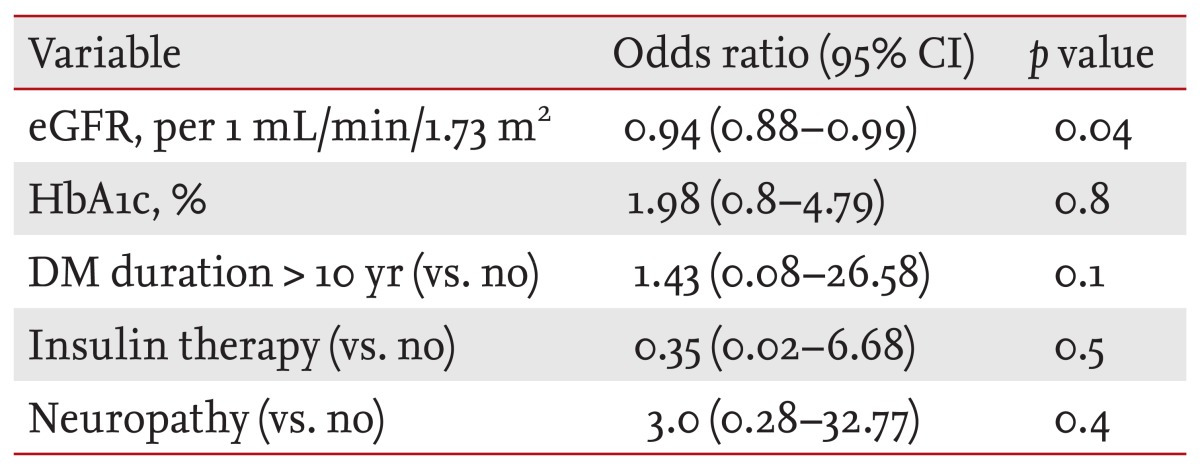
The mean patient age was 60 ± 10 years; the IPSS score was 3.7 ± 3.3; and the diabetes duration was 11.9 ± 7.8 years. Seven of the 42 patients (16.7%) had a PVR > 50 mL. The presence of overt proteinuria or microalbuminuria was associated with an increased risk of a PVR > 50 mL (p < 0.01). Patients with a PVR > 50 mL had a significantly lower estimated glomerular filtration rate (eGFR) compared with those with a PVR ≤ 50 mL (59.2 ± 27.1 mL/min/1.73 m2 vs. 28.7 ± 23.3 mL/min/1.73 m2; p < 0.001). Multivariate logistic analysis revealed that a lower eGFR (odds ratio, 0.94; 95% confidence interval, 0.88 to 0.99; p = 0.04) was a significant risk factor for a PVR > 50 mL.
Conclusions
Patients with diabetic nephropathy had a significantly higher PVR and a lower eGFR was associated with an abnormal PVR.
INTRODUCTION
Diabetic cystopathy (DC) is an important long-term complication in patients with diabetes mellitus (DM); it occurs in 30% to 80% of diabetics [1,2,3,4]. The prevalence of DC is not related to patient age or gender; however, it increases with the duration of diabetes [1,5]. DC develops insidiously, and does not appear until the disease is at an advanced stage [2]. The classical characteristics of DC are impaired or loss of filling sensation, increased bladder capacity, decreased detrusor contractility, and increased post-voiding residual (PVR) urine [1,6,7]. Patients who develop PVR are prone to recurrent urinary tract infections and a deterioration in renal function via obstructive effects [2]. Clinical and experimental studies revealed that polyneuropathy induces DC and that it predominantly affects sensory and autonomic nerve fibers [6,8,9].
There is a well-established correlation between the duration of DC and the presence of peripheral neuropathy. In addition, several authors have suggested that DC-induced bladder dysfunction has harmful effects on renal function [2,5,10]. However, the studies that assessed the correlation between the degree of DC and diabetic nephropathy were unsatisfactory [10,11]. In one study, 34% of the patients with DC had diabetic nephropathy [1]. Therefore, our study assessed the correlation between PVR and the degree of diabetic nephropathy and other microvascular complications in type 2 diabetic patients with no voiding symptoms.
METHODS
Patients and methods
The study included 42 patients with type 2 diabetes (19 males, 23 females; age, 40 to 85 years) and no complaints of voiding problems. All patients were followed regularly at our outpatient clinic between July 1, 2008 and June 30, 2009. To test for the presence of lower urinary tract symptoms objectively, the International Prostate Symptom Score (IPSS) of all patients was assessed, and those with a score < 12 were enrolled [12,13]. The exclusion criteria were: (1) end-stage renal disease; (2) any medication known to interfere with bladder or sphincter function, such as calcium channel blockers, anticholinergics, α- and β-adrenergic agonists, narcotics, antidepressants, antipsychotics, and diuretics; (3) acute metabolic complications of DM including ketoacidosis or a hyperosmolar hyperglycemic state; (4) suprapontine or suprasacral lesions or any root lesion of the sacral and lumbar outflow tracts, and diseases related to a peripheral neuropathy other than DM; (5) a history of benign prostate hyperplasia or stress incontinence; and (6) previous genitourinary surgery that might affect vesicourethral function.
The patient evaluations consisted of a detailed history of the duration of diabetes, type of treatment, laboratory findings, concomitant diabetic retinopathy and nephropathy, and urodynamic investigations. Diabetic retinopathy was evaluated by an ophthalmologist. The findings were classified as normal, nonproliferative, or proliferative diabetic retinopathy. To evaluate diabetic neuropathy, pin-prick, thermal, and vibration sensations were evaluated, and neurologists performed electroneuromyography. The spot urine microalbumin-to-creatinine and urinary protein-to-creatinine ratios were checked in all patients. Nephropathy was defined as a modification of diet in renal disease-estimated glomerular filtration rate (eGFR) [14] < 60 mL/min/1.73 m2 or a urinary microalbumin-to-creatinine ratio > 30 mg/g [15]. A urologist performed the urological evaluations, and PVR was measured using a bladder scan (Cube flow, Mcube Technology, Seoul, Korea). PVR was used as a diagnostic sign of DC. A PVR > 50 mL on two consecutive voids with a total urine volume ≥ 150 mL was considered to be abnormal [16]. Informed consent was obtained from all patients, and the Institutional Review Board approved the study protocol.
Statistical analysis
The data are presented as means ± standard deviations, and the differences between groups were analyzed using nonparametric Mann-Whitney U tests. Categorical variables were analyzed using Fisher exact tests. The correlation between the baseline eGFR and PVR was evaluated using Spearman correlation analysis. Logistic regression analysis was used to assess the influence of different covariates on a PVR > 50 mL. Variables that were significantly associated with a PVR < 50 mL in the univariate analyses and in previous studies were included in the multivariate analysis: eGFR and hemoglobin A1c (HbA1c) were used as continuous variables, and diabetic neuropathy, insulin therapy, and the duration of diabetes were categorical variables. A value of p < 0.05 was considered to indicate statistical significance. The statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
The study enrolled 19 males and 23 females; the mean patient age was 60 ± 1 years. The IPSS scores ranged from 0 to 11 (mean, 3.7). The mean duration of diabetes was 11.9 years (range, 1 to 34). When the association between PVR and microvascular complications was assessed (Fig. 1), patients with neuropathy (28.6%) and retinopathy (54.8%) had a higher PVR than did those without neuropathy or retinopathy, but the difference was not significant. Specifically, patients with nephropathy had a significantly higher PVR than those without nephropathy (59.7 ± 100.1 mL vs. 19.8 ± 13.5 mL, p = 0.04).
Seven of the 42 patients (16.7%) had a PVR > 50 mL. Table 1 presents the clinical characteristics of the patients, stratified according to a PVR > 50 mL. The prevalence of female gender, a DM duration > 10 years, IPSS, and HbA1c levels did not differ significantly between the groups with a PVR > 50 mL and ≤ 50 mL. Only eGFR was significantly lower in patients with a PVR > 50 mL. When we evaluated the prevalence of a PVR > 50 mL according to proteinuria (Fig. 2), no patients without proteinuria had a PVR > 50 mL, while one patient (16.7%) with microalbuminuria and four patients (33.3%) with proteinuria had a PVR > 50 mL. The correlation between the baseline eGFR and PVR was inverse and significant (r = -0.33, p = 0.03). Multivariate logistic regression analysis was used to evaluate the risk factors for a PVR > 50 mL, and the results are listed in Table 2. The presence of neuropathy, insulin therapy, a higher HbA1c, and a DM duration > 10 years were not significant risk factors for a PVR > 50 mL. However, a lower eGFR (odds ratio, 0.94; 95% confidence interval, 0.88 to 0.99; p = 0.04) was a significant risk factor for a PVR > 50 mL.
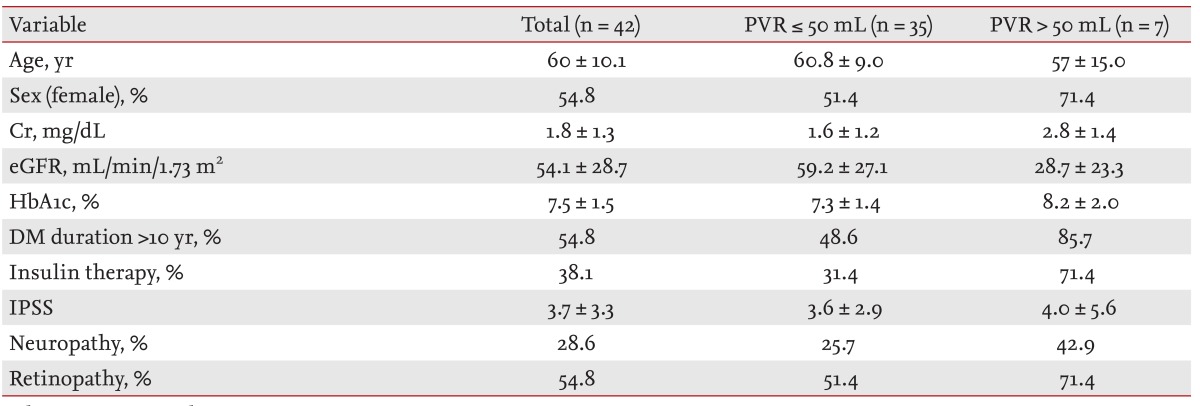
Clinical characteristics of patients with post-voiding residual (PVR) urine volume > 50 mL and with PVR ≤ 50 mL
DISCUSSION
This study evaluated the association between nephropathy and PVR in 42 diabetics with low IPSS scores and no voiding symptoms. DC has an insidious onset and progression, with minimal symptomatology. If the presence of DC could be predicted during the asymp tomatic phase, appropriate preventive and therapeutic approaches could be taken. Our data revealed that a decreased eGFR is an independent risk factor for a PVR > 50 mL. Therefore, measuring PVR could predict DC early, and allow therapeutic approaches to diabetic patients with a decreased eGFR.
The diagnostic criteria for nephropathy are equivocal, and some reports have demonstrated an association between nephropathy and cystopathy. In one study of 17 diabetics with diabetic nephropathy but no voiding symptoms, there was no relationship between a PVR > 150 mL (47%) and a decline in kidney function [17]. Another study of 66 diabetics revealed no significant association between PVR and nephropathy [18]. However, a study of 54 diabetics with lower urinary tract symptoms reported that microalbuminuria is a risk factor for a PVR > 100 mL [19]. Norden et al. [10] reported that diabetics with consistent or large residual volumes exhibited a rapid decline in eGFR. In the current study, the prevalence of a PVR > 50 mL was increased in the presence of proteinuria and PVR was inversely correlated with eGFR (r = -0.3, p = 0.04). In addition, multivariate analysis revealed that eGFR is a risk factor for a PVR 50 mL. Although the data remain controversial, there could be an association between the residual volume and renal impairment.
Previous studies showed that the prevalence of cystopathy in diabetic patients with peripheral neuropathy was between 75% and 100% [1,3,5]. In addition, DC was associated with autonomic neuropathy [8]. In a urodynamic study of 66 diabetics with no voiding symptoms, Esteghamati et al. [18] reported that the presence of somatic peripheral neuropathy was associated with a 5-fold increased risk of having a low flow rate. In our study, in which the prevalence of DC in asymptomatic patients was 16.7%, patients with neuropathy had a higher PVR than did those without neuropathy (64.9 mL vs. 39 mL). However, the difference was not significant. Although diabetic retinopathy was not related to DC in this work, a previous study reported that it was a risk factor for the development of PVR [19]. We found that patients with retinopathy had a higher PVR than did those without neuropathy (62.3 mL vs. 27.1 mL), but the difference was not significant.
Early studies of cystopathy demonstrated that the frequency of cystopathy was similar between genders [2]. In addition, there was no significant correlation between the dose of insulin and the frequency of cystopathy [3]. However, a longer duration of diabetes was related to a greater incidence of cystopathy [20]. In the current study, the prevalence of female gender, a diabetes duration > 10 years, and the use of insulin therapy did not differ between groups.
There were several limitations to this study. First, no follow-up data were available; therefore, the relationship between cystopathy and the progression of kidney function could not be evaluated. Second, we used only the presence of residual urine volume as a diagnostic sign of DC. However, the presence of residual urine is a fundamental factor for differentiating between the incipient stage of a neurogenic bladder and the advanced stage of DC [6,10]. Third, the small sample size might have low power for validating a significant association between some of the parameters evaluated in this study. Therefore, further studies with larger sample sizes are needed assess any potential associations.
The results of this study suggest that a decreased eGFR is associated with an increased PVR. The occurrence of residual urine is an important sign of DC, which can be asymptomatic for many years. Further research should evaluate whether measuring PVR and therapeutic approaches at an earlier stage of DC could have beneficial effects on the progression of DC or nephropathy.
KEY MESSAGE
Diabetic cystopathy (DC) is a frequent complication of diabetes mellitus.
There was a negative association between estimated glomerular f iltration rate (eGFR) and post-voiding residual (PVR) and a lower eGFR was a significant risk factor for a PVR urine volume > 50 mL.
Patients with diabetic nephropathy should have PVR measured to screen for DC, even if the patients do not complain of any voiding problems.
Notes
No potential conflict of interest relevant to this article was reported.