Clinical impact of routine follow-up coronary angiography after second- or third-generation drug-eluting stent insertion in clinically stable patients
Article information
Abstract
Background/Aims
In the bare-metal stent era, routine follow-up coronary angiography (RFU CAG) was used to ensure stent patency. With the advent of drug-eluting stents (DESs) with better safety and efficacy profiles, RFU CAG has been performed less often. There are few data on the clinical impact of RFU CAG after second- or third-generation DES implantation in clinically stable patients with coronary artery disease; the aim of this study was to examine this issue.
Methods
We analyzed clinical outcomes retrospectively of 259 patients who were event-free at 12-month after stent implantation and did not undergo RFU CAG (clinical follow-up group) and 364 patients who were event-free prior to RFU CAG (angiographic follow-up group). Baseline characteristics were compared between the groups.
Results
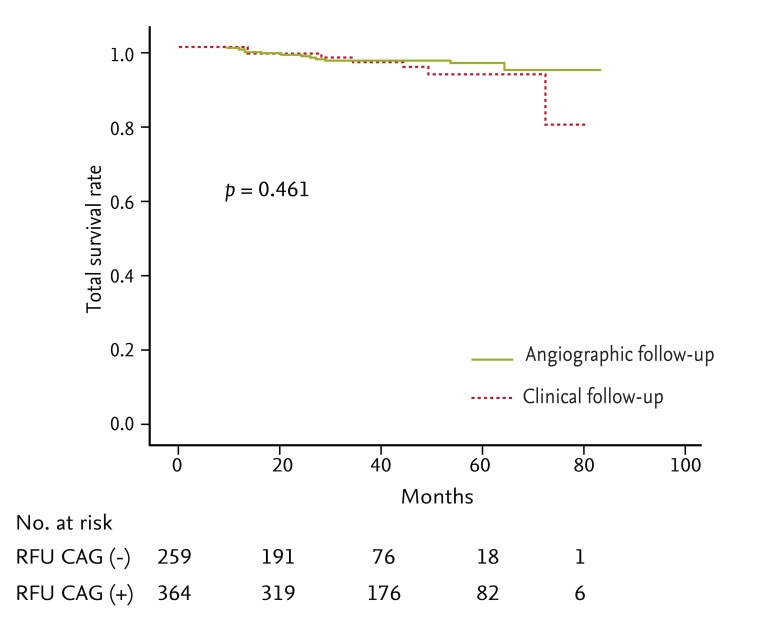
The Kaplan-Meier estimated total survival and major adverse cardiac event (MACE)-free survival did not differ between the groups (p = 0.100 and p = 0.461, respectively). The cumulative MACE rate was also not different between the groups (hazard ratio, 0.85; 95% confidence interval, 0.35 to 2.02). In the angiographic follow-up group, 8.8% revascularization was seen at RFU CAG.
Conclusions
RFU CAG did not affect long-term clinical outcome after second- or third-generation DES implantation in clinically stable patients.
INTRODUCTION
With the advent of thin-strut designs and biocompatible polymers, second- or third-generation drug-eluting stents (DESs) have been demonstrated to have improved safety and efficacy compared with first-generation DESs [1,2,3,4,5,6]. Nonetheless, "routine follow-up coronary angiography (RFU CAG)" is still performed in some countries, including Korea, after second- or third-generation DES implantation [7]. RFU CAG is usually performed at 6 to 12 months after stent implantation for randomized stent trials or at the clinician's discretion for the early detection and treatment of in-stent restenosis (ISR) or de novo lesions in high-risk patients. Several studies on the clinical impact of RFU CAG after implantation of bare-metal stents (BMSs) or first-generation DESs reported that RFU CAG did not affect long term clinical outcome and caused more revascularization in angiographic follow-up group [8,9,10,11,12,13,14,15,16]. Despite the widespread adoption of second- and third-generation DESs, there are limited data on the clinical impact of RFU CAG after second- or third-generation DES implantation in clinically stable patients with coronary artery disease. Thus, we analyzed single-center data retrospectively and compared clinical outcomes between clinical follow-up and angiographic follow-up groups after implantation of second- or third-generation DESs. The clinical follow-up group was defined as those who were event-free at 12 months after stent implantation and did not undergo RFU CAG, and the angiographic follow-up group was defined as those who were event-free prior to RFU CAG.
METHODS
Patient selection
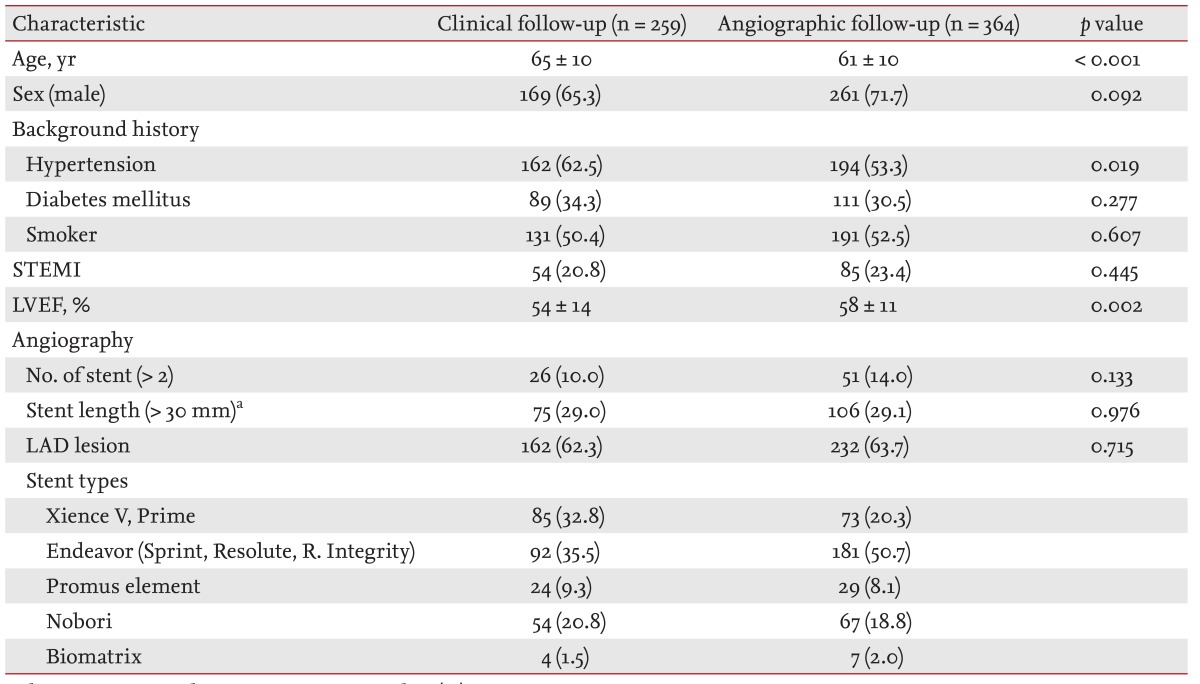
An observational study was conducted on patients receiving second- or third-generation DES at Hallym University Kangnam Sacred Heart Hospital, Hallym University Medical Center, Korea, between January 2007 and December 2012. From 920 initially screened patients, we excluded 271 who had experienced events (death, ischemic events, follow-up CAG due to symptoms or abnormal results in noninvasive tests, revascularization, or loss to follow-up) during the early term (the first 12 months after stent implantation in the clinical follow-up group and until RFU CAG in the angiographic follow-up group) and also excluded 26 further patients with late-term loss to follow-up. Overall, 623 patients were eligible to participate; of them 259 patients were event-free at 12 months after stent implantation and did not undergo RFU CAG (clinical follow-up group) and 364 patients were event-free prior to RFU CAG (angiographic follow-up group). RFU CAG was performed for randomized stent trials or at the clinician's decisions. The stent types used were second- or third-generation DESs: Xience V, Xience Prime, Endeavor Sprint, Endeavor Resolute, Resolute Integrity, Promus Element, Nobori, and Biomatrix stents.
End point
The primary endpoint was major adverse cardiac events (MACEs). MACE included cardiovascular death, myocardial infarction (MI), ischemic heart failure requiring hospitalization (IHFRH), and revascularization. The definition of MI was a combination of changes in cardiac biomarkers and supporting information, derived from the clinical presentation, electrocardiographic changes, or the results of myocardial or coronary artery imaging. The definition of IHFRH was hospitalization, combined with clinical symptoms and signs of heart failure and no other noncardiac or cardiac etiology of heart failure, with the exception of ischemic heart disease, was demonstrable. Because the aim of this study was to evaluate the clinical impact of RFU CAG, which provides details not only on the stented segment but also on the entire vascular bed, we also included target vessel revascularization (TVR) and non-target vessel revascularization (NTVR) besides target lesion revascularization (TLR) in the revascularization of MACE. However, we did not include revascularization at RFU CAG in the revascularization of MACE because the main purpose of this study was to compare the long-term clinical outcome of the angiographic follow-up group with that of the clinical follow-up group who did not undergo RFU CAG. Causes of noncardiac death included cancer, bleeding, and infection.
Statistical analysis
The SPSS version 21.0 (IBM Co., Armonk, NY, USA) was used to analyze the results. All continuous variables are described as means ± SD. All categorical variables are described using absolute and relative frequency distributions. Comparisons between groups used unpaired t tests for continuous variables and chi-square tests for discrete variables. Survival curves were generated with the Kaplan-Meier method and compared using log-rank tests. We also used hazard ratios from Cox regression models to quantify relative risks of MACE. To identify independent predictors for MACE, multivariable logistic regression analysis was performed. A p < 0.05 was considered to indicate statistical significance.
RESULTS
Patients
The baseline characteristics of the two groups were not different, except older age and lower ejection fraction in the clinical follow-up group. The proportions of DM, ST-segment-elevation myocardial infarction, and smoking patients were about 30%, 20%, and 50% in both groups. The proportion of patients with stent lengths longer than 30 mm was about 30% and proportion of patients with a left anterior descending coronary lesion was about 60% in both groups. The Endeavor stents were inserted more often in the angiographic follow-up group and Xience stents were used more often in the clinical follow-up group (Table 1). There was no difference in medication history, including antiplatelet agent and statin use, between the groups.
Routine follow-up coronary angiography
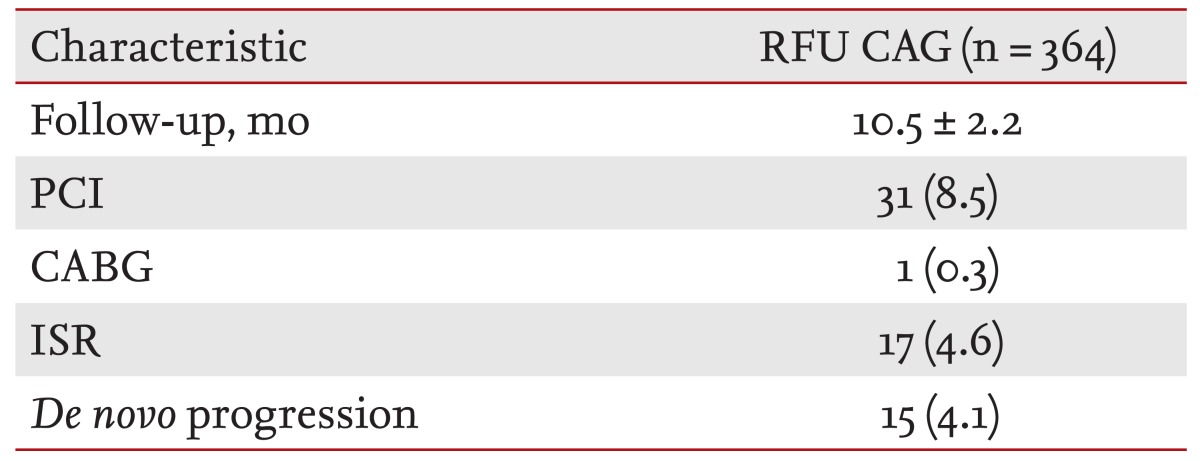
RFU CAG was performed in 364 patients and the mean follow-up duration of RFU CAG was 10.5 months. The revascularization rate at RFU CAG was 8.8%. More than half of revascularization was due to ISR (Table 2).
Death and composite cardiac end point
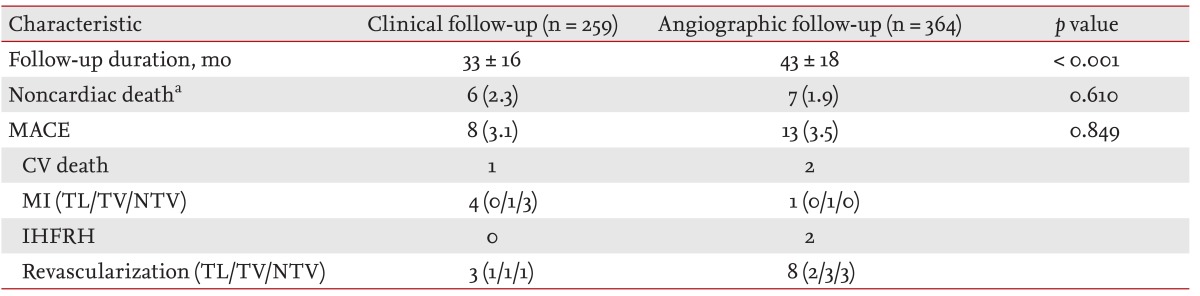
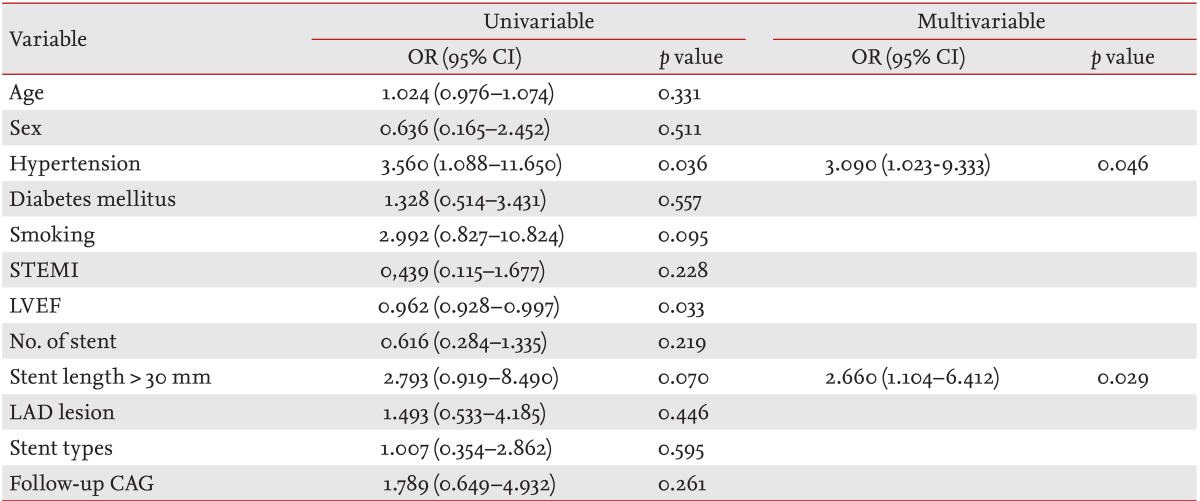
The mean clinical follow-up durations were 33 months in clinical follow-up group and 43 months in angiographic follow-up group. The Kaplan-Meier estimated total survival and MACE-free survival did not differ between the groups (p = 0.100 and p = 0.461, respectively) (Figs. 1 and 2). The cumulative MACE rate was also not different between the groups (hazard ratio, 0.85; 95% confidence interval, 0.35 to 2.02) (Fig. 3). MACE included one cardiovascular death, four MI, and three revascularizations in the clinical follow-up group, and two cardiovascular deaths, one MI, two IHFRH, and eight revascularizations in the angiographic follow-up group. Most of the culprit lesions in revascularization and MI in both groups were target vessel or nontarget vessel lesions, rather than target lesions (86% in the clinical follow-up group versus 78% of the angiographic follow-up group) (Table 3). A multivariable logistic regression analysis revealed that the only significant predictors of MACE were hypertension and stent length more than 30 mm after adjusting for other factors (Table 4). Follow-up CAG was not a significant predictor of MACE.

Kaplan-Meier total survival in angiographic followup versus clinical follow-up group. RUF CAG, routine followup coronary angiography.
Kaplan-Meier survival free of major adverse cardiac event in angiographic follow-up versus clinical follow-up group. RUF CAG, routine follow-up coronary angiography.

Rates for relative risk of major adverse cardiac event in angiographic follow-up versus clinical follow-up group. CI, confidence interval.
DISCUSSION
The main finding of this study was that when a patient has not developed ischemic signs or symptoms at 12 months after second- or third-generation DES implantation, there is no need for a RFU angiography, with its added cost and procedural risk. Another issue is that RFU CAG causes a relatively high revascularization rate in patients with no ischemic signs or symptoms, via a phenomenon known as the "oculostenotic reflex" [14].
We performed this study to evaluate the clinical utility of mid-term follow-up CAG after second- or third-DES implantation. We assumed that when a patient was event-free at 12 months after second- or third-DES implantation, there would be no need for RFU angiography. This assumption was based on the findings of prior studies that nearly two-thirds of ischemic events after stent implantation occurred during the first 12 months [17,18,19]. Several studies on the clinical impact of RFU CAG after BMS or first-generation DES implantation showed that RFU CAG did not affect long-term clinical outcome and the follow-up angiography group suffered a twofold higher rate of revascularization, primarily due to revascularization at follow-up angiography [7,8,9,10,11,12,13,14,15,16]. These studies described that RFU angiography caused a high revascularization rate in patients who otherwise would not had undergone CAG. Revascularization at follow-up angiography was due to both TLR and TVR. However, with markedly lower rates of restenosis in the DES era, TLR tended to decrease and TVR had become a major proportion of revascularization cases [15]. One study reported that early treatment of de novo lesions as well as ISR lesions in the angiographic follow-up group was largely offset by revascularization performed later in the clinical follow-up group [15]. They described that late catch-up occurred in the clinical follow-up group and the revascularization rates were comparable between the angiographic follow-up and clinical follow-up groups by 3 years. Although there was no statistical significance, similar tendencies were observed in other studies [9,10]. Moreover, if we include the revascularizations at RFU CAG in MACE, as in other studies, then a tendency for initial high revascularization in the angiographic follow-up group and late catch-up in the clinical follow-up group was also noted in our study. This early treatment effect of follow-up angiography may provide a rationale for performing follow-up angiography, but needs further research and a cost-effectiveness analysis. Considering the broad definition of revascularization in this study, including TLR, TVR, and NTVR, MACE rate of 3.1% and 3.5% in the groups in this study were lower than those in landmark studies: 5% to 7% [20,21,22]. However, the difference could also be attributable largely to the difference between the use of all-cause mortality in other studies versus cardiovascular mortality in this study. Because the MACE definition is heterogeneous and there is no consensus definition among the studies, caution is needed when comparing "MACE rates" between studies [23]. Another possible explanation for the lower MACE rate may be related to retrospective design of this study and the patients lost to follow-up may have undergone serious ischemic events and were lost to follow-up for that reason.
There are several limitations to this study. It was a single-center, retrospective study and the sample size was modest. Also, the two groups were inhomogeneous because this was not a prospective randomized study. However, we believe that our findings provide valuable insight on the role of RFU CAG after second- or third-generation DES implantation. In conclusion, we found that RFU CAG did not affect the long-term clinical outcome after second- or third-generation DES implantation in clinically stable patients.
KEY MESSAGE
Routine follow-up coronary angiography (CAG) did not affect long-term clinical outcome after second- or third-generation drug-eluting stent implantation in clinically stable patients.
Routine follow-up CAG may cause a revascularizations in clinically stable patients who otherwise would not had undergone CAG, via a phenomenon known as the "oculostenotic reflex."
Notes
No potential conflict of interest relevant to this article was reported.