Efficacy and safety of tofacitinib for active rheumatoid arthritis with an inadequate response to methotrexate or disease-modifying antirheumatic drugs: a meta-analysis of randomized controlled trials
Article information
Abstract
Background/Aims
The aim of this study was to assess the efficacy and safety of tofacitinib (5 and 10 mg twice daily) in patients with active rheumatoid arthritis (RA).
Methods
A systematic review of randomized controlled trials (RCTs) that examined the efficacy and safety of tofacitinib in patients with active RA was performed using the Medline, Embase, and Cochrane Controlled Trials Register databases as well as manual searches.
Results
Five RCTs, including three phase-II and two phase-III trials involving 1,590 patients, met the inclusion criteria. The three phase-II RCTs included 452 patients with RA (144 patients randomized to 5 mg of tofacitinib twice daily, 156 patients randomized to 10 mg of tofacitinib twice daily, and 152 patients randomized to placebo) who were included in this meta-analysis. The American College of Rheumatology 20% response rate was significantly higher in the tofacitinib 5- and 10-mg groups than in the control group (relative risk [RR], 2.445; 95% confidence interval [CI], 1.229 to 4.861; p = 0.011; and RR, 2.597; 95% CI, 1.514 to 4.455; p = 0.001, respectively). The safety outcomes did not differ between the tofacitinib 5- and 10-mg groups and placebo groups with the exception of infection in the tofacitinib 10-mg group (RR, 2.133; 95% CI, 1.268 to 3.590; p = 0.004). The results of two phase-III trials (1,123 patients) confirmed the findings in the phase-II studies.
Conclusions
Tofacitinib at dosages of 5 and 10 mg twice daily was found to be effective in patients with active RA that inadequately responded to methotrexate or disease-modifying antirheumatic drugs, and showed a manageable safety profile.
INTRODUCTION
Rheumatoid arthritis (RA) is a systemic autoimmune disease characterized by chronic synovial joint inflammation that leads to disability and loss of quality of life [1,2]. Disease-modifying antirheumatic drugs (DMARDs) have been used to decrease inflammation, delay bone erosion, and improve functional ability in patients with RA.
Intracellular pathways that include Janus kinases (JAKs) are critical to immune cell activation, proinflammatory cytokine production, and cytokine signaling [3]. Tofacitinib (CP-690,550) is a novel, orally administered JAK inhibitor [4]. Tofacitinib selectively inhibits JAK-1, JAK-2, and JAK-3, with functional cellular specificity for JAK-1 and JAK-3 over JAK-2 [5,6]. Inhibition of JAK-3, in combination with JAK-1, blocks signaling through interleukin-2 (IL-2), IL-4, IL-7, IL-9, IL-15, and IL-21, which are integral to lymphocyte function and may thus modulate the immune response [6]. Tofacitinib subsequently modulates adaptive and innate immunity [6].
Several clinical trials have been performed to evaluate the efficacy and safety of tofacitinib in patients with active RA that showed an inadequate response to DMARDs or methotrexate (MTX) [7,8,9,10,11]. The Food and Drug Administration approved tofacitinib oral tablets as a second-line treatment for moderate-to-severe RA on 6 November, 2012, and the recommended dosage of tofacitinib is 5 mg twice daily [12]. Meta-analyses integrate previous research, increase statistical power, and provide a more accurate estimate of the effect magnitude by pooling the results of independent analyses. The aim of the present study was to increase the precision and accuracy of estimates of the efficacy and safety of tofacitinib at 5 and 10 mg twice daily in patients with active RA by performing a meta-analysis of randomized controlled trials (RCTs) and systematically reviewing the available data [13,14].
METHODS
Identification of eligible studies and data extraction
We performed an exhaustive search for studies that examined the efficacy and safety of tofacitinib in patients with active RA that had inadequately responded to DMARDs or MTX. Literature searches were performed using the Medline, Embase, and Cochrane Controlled Trials Register databases to identify available articles published up to February 2013. The following keywords and subject terms were used in the searches: "tofacitinib," "rheumatoid arthritis," and "RA." All references in the studies were reviewed to identify additional works not included in the electronic databases. RCTs were included if they met the following criteria: (1) the study compared tofacitinib with placebo for the treatment of RA; (2) the study provided endpoints for the clinical efficacy and safety of tofacitinib; and (3) the study included patients diagnosed with RA based on the American College of Rheumatology (ACR) criteria for RA. The exclusion criteria were as follows: (1) the study included duplicate data; and (2) the study did not contain adequate data for inclusion.
The efficacy outcomes were as follows: (1) number of patients who achieved an ACR 20% response rate (ACR20); (2) number of tender and swollen joints; (3) pain (visual analog scale); (4) patients' and physicians' global assessments of disease activity; (5) health assessment questionnaire (HAQ) score; and (6) C-reactive protein level. The safety outcomes were the number of patients who withdrew from the study because of adverse events (AEs), serious AEs, serious infections that were considered to be related to the medication, total number of infections, and abnormal liver function test results. The following information was extracted from each study: first author, year of publication, country in which the study was conducted, dose of tofacitinib, length of follow-up, primary outcomes for efficacy and safety, and mean and standard deviation of changes from baseline. We quantified the methodological qualities of the four studies using Jadad scores. We conducted a systematic review and meta-analysis in accordance with the guidelines provided by the PRISMA statement. Data were extracted from the original studies by two independent reviewers. Discrepancies between the reviewers were resolved by consensus or by a third reviewer.
Evaluation of publication bias
Funnel plots are normally used to detect publication bias. However, because funnel plots require a range of studies with different sizes and subjective judgments, we evaluated publication bias using Egger's linear regression test [15]. Egger's test measures funnel plot asymmetry using a natural logarithm scale of relative risk (RR).
Evaluations of statistical associations
For continuous data, the results are presented as weighted mean differences (WMDs), and RR was calculated for dichotomous data. We assessed within- and between-study variations and heterogeneity using Cochran's Q statistic [16]. The heterogeneity test was used to assess the null hypothesis that all studies evaluated the same effect. When a statistically significant Q statistic (p < 0.10) indicated heterogeneity across studies, a random-effects model was used for the meta-analysis [17]; if not, a fixed-effects model was used. The fixed-effects model assumes that all studies estimate the same underlying effect and considers only within-study variation [16]. We quantified the effect of heterogeneity using the formula I2 = 100% × (Q - df) / Q [18], where I2 indicates the degree of inconsistency between studies and determines whether the percentage total variation across studies is due to heterogeneity rather than chance. I2 ranges from 0% to 100%; I2 values of 25%, 50%, and 75% are referred to as low, moderate, and high estimates, respectively [18]. Statistical manipulations were undertaken using computer software (Comprehensive Meta-Analysis, Biosta, Englewood, NJ, USA).
RESULTS
Studies included in the meta-analysis
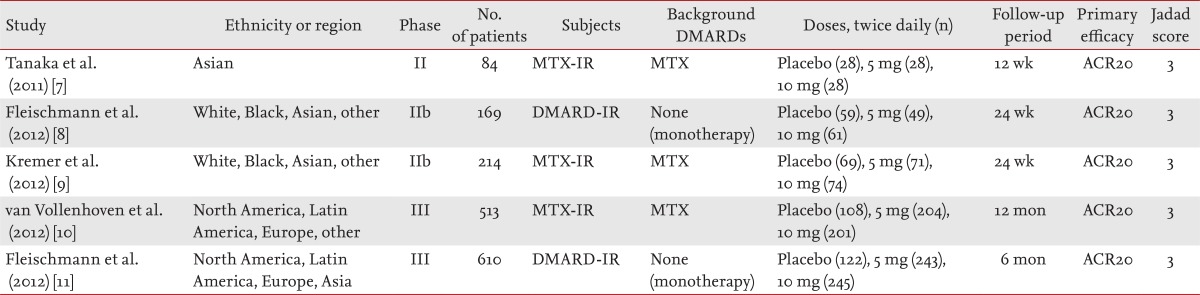
Twenty-one studies were identified by electronic or manual searches, and seven were selected for a full-text review based on the title and abstract [7,8,9,10,11,19,20]. However, two of the seven studies were excluded because they contained duplicate data [19,20]. Thus, five RCTs met the inclusion criteria: three phase-II [7,8,9] and two phase-III trials [10,11] involving 1,590 patients. The relevant features of the studies included in the meta-analysis are provided in Table 1. The patients in three studies received both tofacitinib and MTX, while the patients in the remaining two studies received tofacitinib alone. The follow-up period ranged from 12 weeks to 12 months, and all studies had a Jadad score of 3 (Table 1). We performed a meta-analysis of three phase-II trials that reported data on 144 patients randomized to receive tofacitinib 5 mg, 156 randomized to receive tofacitinib 10 mg, and 152 randomized to receive placebo [7,8,9].
Meta-analysis of the efficacy of tofacitinib at 5 and 10 mg twice per day for RA in phase-II trials
The meta-analysis of efficacy was performed at 12 weeks because all data on the efficacy outcomes among the three studies were available at 12 weeks [7,8,9]. The ACR20 response rate was significantly higher in the tofacitinib 5-mg group than in the control group (RR, 2.445; 95% confidence interval [CI], 1.229 to 4.861; p = 0.011) (Table 2, Fig. 1). Tofacitinib was associated with a significantly lower HAQ score than was placebo in patients with RA (WMD, -0.341; 95% CI, -0.455 to -0.226; p < 1.0 × 10-8) (Table 2, Fig. 2). Similarly, the ACR20 response rate was significantly higher in the tofacitinib 10-mg group than in the control group (RR, 2.597; 95% CI, 1.514 to 4.455; p = 0.001) (Table 2, Fig. 1). Tofacitinib at 10 mg twice per day was associated with a significantly lower HAQ score than was placebo in patients with RA (WMD, -0.344; 95% CI, -0.461 to -0.227; p < 1.0 × 10-8) (Table 2, Fig. 2). Statistically significant improvements were observed in the tofacitinib 5- and 10-mg groups compared with the control group for all efficacy outcomes, including the number of tender and swollen joints, pain, patients' and physicians' global assessments of disease activity, HAQ score, and C-reactive protein level (Table 2).
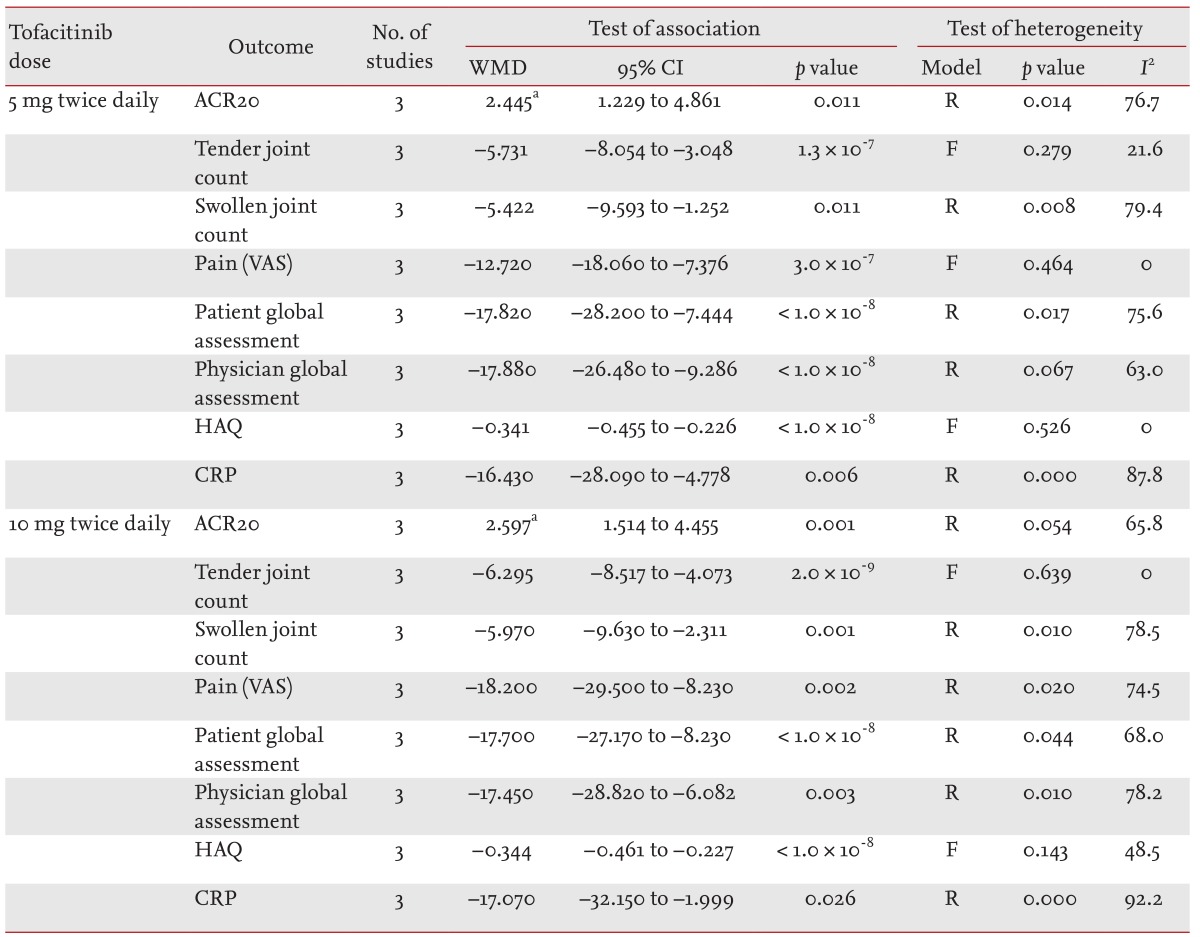
Meta-analysis of randomized controlled trials on the efficacy of tofacitinib in patients with active rheumatoid arthritis

Meta-analysis of the efficacy of tofacitinib at (A) 5 mg and (B) 10 mg twice per day on the American College of Rheumatology 20% response rate in patients with rheumatoid arthritis. CI, confidence interval.
Two RCTs included data at the 24-week follow-up. Both studies showed a significant improvement according to the efficacy outcome, including the ACR20 response rates in both the tofacitinib 5- and 10-mg groups, when the data were analyzed at 24 weeks.
Meta-analysis of the safety of tofacitinib for RA in phase-II trials
The number of patients who withdrew from the study because of AEs did not differ between the tofacitinib 5- and 10-mg groups and the placebo group (RR, 1.091; 95% CI, 0.387 to 3.081; p = 0.869 and RR, 1.285; 95% CI, 0.481 to 3.430; p = 0.617, respectively) (Table 3, Fig. 3). The incidence of serious AEs did not differ between the tofacitinib 5- and 10-mg groups and the placebo group (RR, 1.430; 95% CI, 0.293 to 6.971; p = 0.658 and RR, 1.035; 95% CI, 0.177 to 6.045; p = 0.970, respectively) (Table 3). The incidence of serious infections and abnormal liver function test results did not differ between the tofacitinib 5- and 10-mg groups and the placebo group (Table 3). However, the infection rate was significantly higher in the tofacitinib 10-mg group than in the placebo group (RR, 2.133; 95% CI, 1.268 to 3.590; p = 0.004) (Table 3).
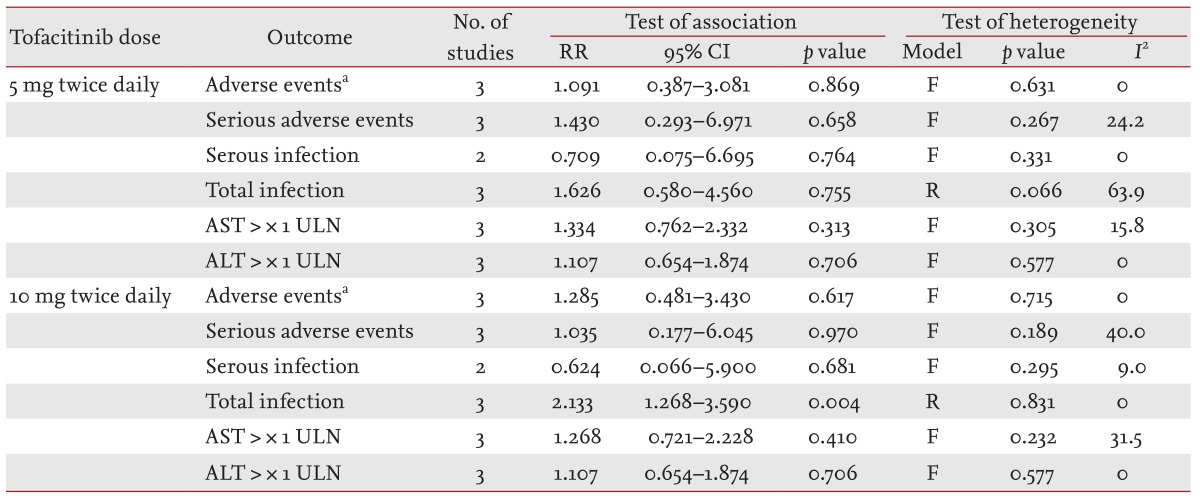
Meta-analysis of randomized controlled trials on the safety of tofacitinib in patients with active rheumatoid arthritis
Heterogeneity and publication bias
For most of the meta-analyses, between-study heterogeneity was conducted on the efficacy of tofacitinib at 5 and 10 mg twice daily. However, no between-study heterogeneity was found for analyses conducted on the safety of tofacitinib with the exception of the analysis of total infection. It was difficult to correlate the funnel plot, which is usually used to detect publication bias, because the number of studies included in the analysis was too small. However, no evidence of publication bias was obtained (Egger's regression test p > 0.1).
Efficacy and safety of tofacitinib in phase-III studies
Two phase-III RCTs on tofacitinib in patients with RA were evaluated [10,11]. The studies had different study designs; thus, we did not perform a meta-analysis. Van Vollenhoven et al. [10] performed a study in which 513 patients with an incomplete response to MTX were randomly assigned to 5 mg of tofacitinib twice daily, 10 mg of tofacitinib twice daily, or placebo for 12 months. At month 3, patients in the placebo group who did not have a 20% reduction in the number of swollen and tender joints from baseline were switched to either 5 or 10 mg of tofacitinib twice daily [10]. At month 6, all patients still receiving placebo were switched to tofacitinib. At month 6, the ACR20 response rates were higher among patients receiving 5 or 10 mg of tofacitinib (51.5% and 52.6%, respectively) than among those receiving placebo (28.3%, p < 0.001). AEs occurred more frequently with tofacitinib than with placebo, and pulmonary tuberculosis developed in two patients in the 10-mg tofacitinib group.
Fleischmann et al. [11] performed a phase-III, double-blind, placebo-controlled, parallel-group, 6-month study in which 610 patients were randomly assigned in a 4:4:1:1 ratio to 5 mg of tofacitinib twice daily, 10 mg of tofacitinib twice daily, placebo for 3 months followed by 5 mg of tofacitinib twice daily, or placebo for 3 months followed by 10 mg of tofacitinib twice daily. At month 3, the tofacitinib groups had a higher ACR20 response rate than did the placebo groups (59.8% in the 5-mg tofacitinib group and 65.7% in the 10-mg tofacitinib group vs. 26.7% in the combined placebo groups, p < 0.001). Serious infections developed in six patients who were receiving tofacitinib. Taken together, the findings in both of the phase-III trials (1,123 patients) confirmed the results of the meta-analysis of the phase-II RCTs of tofacitinib and showed that tofacitinib at dosages of 5 and 10 mg twice per day provided a clinical benefit in trials of patients with active RA with or without background MTX treatment. The phase-III RCTs revealed the need for studies on the safety of tofacitinib involving larger numbers of patients for longer periods.
DISCUSSION
Multiple cytokines play pivotal roles in the pathogenesis of RA by inducing intracellular signaling, and JAKs are essential for signal transduction [3,21]. Thus, an orally available JAK inhibitor, tofacitinib, has been studied for the treatment of active RA [7,8,9,10,11].
In the present meta-analysis, we combined the clinical data of three phase-II RCTs of tofacitinib at 5 and 10 mg twice per day. The ACR20 response rate was significantly higher in the tofacitinib groups than in the placebo control group, and improvements were observed in each of the individual components of the ACR core set of disease activity measures. The safety outcomes did not differ between the tofacitinib 5 and 10-mg and placebo groups, with the exception of the infection rate in the tofacitinib 10-mg group (RR, 2.133; 95% CI, 1.268 to 3.590; p = 0.004). We did not directly compare the effects of tofacitinib at 5 and 10 mg twice per day. A dose-response relationship was not observed, and whether a dose of 10 mg twice daily is more effective than 5 mg is unclear. Two phase-III trials involving 1,123 patients confirmed the findings found in the meta-analysis of the phase-II studies.
Notably, the trials included in this meta-analysis were performed on patients with active RA that exhibited an inadequate response to DMARDs or MTX. MTX is considered to be the most effective DMARD and has become popular as a first-line drug for RA. However, DMARDs, including MTX, are not effective in many cases. Orally available low-molecular-weight products targeting key molecules in the pathogenesis of RA are attracting increasing attention because they enter the cytoplasm and directly regulate intracellular signals. Our results indicate that orally administered tofacitinib has a potent antirheumatic effect in patients with RA that has inadequately responded to DMARDs. This is clinically meaningful because the patients in the herein-reported studies exhibited insufficient responses to DMARDs. These results suggest that tofacitinib is a useful and valuable treatment in patients with RA that responds inadequately to DMARDs, including MTX.
The meta-analysis is a statistical method that combines quantitative data from various studies to develop a conclusion that has greater statistical power than any individual study. These pooled data are statistically stronger than those of any single study because of the increased numbers of subjects and greater statistical power. Thus, all effect sizes of the studies were in the same direction. However, we performed this meta-analysis of tofacitinib RCTs to increase the precision and accuracy of estimates of the efficacy and safety of tofacitinib in patients with RA.
The present meta-analysis had several shortcomings that should be considered. First, the number of studies included was small, which introduces the potential for sampling errors and publication bias. Although we did not find evidence of bias, it should be kept in mind that publication bias is difficult to exclude with certainty, particularly when the number of incorporated studies is small. Second, no long-term studies were included in the meta-analysis. The follow-up durations of the trials included in the meta-analysis ranged from 12 to 24 weeks. Given the durations and sizes of the studies, it is difficult to draw conclusions regarding the safety of tofacitinib in the RCTs included in this meta-analysis. Actually, in the phase-III trials, the risk of AEs including serious AEs and serious infection was greater in the tofacitinib groups than in the control group [10,11]. Third, radiographic progression was not monitored in any of the included studies, and DMARDs may delay or prevent bone erosion and joint destruction. Therefore, additional studies are required to determine whether tofacitinib delays bone erosion and joint destruction in patients with RA. Fourth, the majority of the included analyses showed between-study heterogeneity, which is of fundamental importance to meta-analyses. When heterogeneity was present in the analyses, we used a random-effects model, which inherently allows for variations in underlying differences in the effect sizes between studies. Furthermore, we do not believe that this heterogeneity could have biased the analysis because all effect sizes were in the same direction.
In conclusion, tofacitinib at dosages of 5 and 10 mg twice daily as monotherapy or in combination with MTX was found to be effective in patients with active RA that demonstrated an inadequate response to DMARDs or MTX and showed a manageable safety profile. Long-term studies are needed to further define the efficacy and safety of tofacitinib at dosages of 5 and 10 mg twice per day in larger numbers of patients with active RA.
KEY MESSAGE
This meta-analysis shows that tofacitinib at dosages of 5 and 10 mg twice daily was effective in patients with active rheumatoid arthritis that had shown an inadequate response to disease-modifying antirheumatic drugs.
Tofacitinib had a manageable safety profile.
Long-term studies are needed to adequately assess the efficacy and safety of tofacitinib.
Acknowledgments
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (A102065).
Notes
No potential conflict of interest relevant to this article was reported.