Effect of anemia correction on left ventricular structure and filling pressure in anemic patients without overt heart disease
Article information
Abstract
Background/Aims
There are few data on the effects of low hemoglobin levels on the left ventricle (LV) in patients without heart disease. The objective of this study was to document changes in the echocardiographic variables of LV structure and function after the correction of anemia without significant cardiovascular disease.
Methods
In total, 34 iron-deficiency anemia patients (35 ± 11 years old, 32 females) without traditional cardiovascular risk factors or cardiovascular disease and 34 age- and gender-matched controls were studied. Assessments included history, physical examination, and echocardiography. Of the 34 patients with anemia enrolled, 20 were followed and underwent echocardiography after correction of the anemia.
Results
There were significant differences between the anemia and control groups in LV diameter, left ventricular mass index (LVMI), left atrial volume index (LAVI), peak mitral early diastolic (E) velocity, peak mitral late diastolic (A) velocity, E/A ratio, the ratio of mitral to mitral annular early diastolic velocity (E/E'), stroke volume, and cardiac index. Twenty patients underwent follow-up echocardiography after treatment of anemia. The follow-up results showed significant decreases in the LV end-diastolic and end-systolic diameters and LVMI, compared with baseline levels. LAVI, E velocity, and E/E' also decreased, suggesting a decrease in LV filling pressure.
Conclusions
Low hemoglobin level was associated with larger cardiac chambers, increased LV, mass and higher LV filling pressure even in the subjects without cardiovascular risk factors or overt cardiovascular disease. Appropriate correction of anemia decreased LV mass, LA volume, and E/E'.
INTRODUCTION
Anemia reduces tissue oxygen delivery and causes a compensatory cardiovascular response. In chronic anemia, the heart undergoes structural changes and develops functional impairment in response to the reduced hemoglobin [1]. Anemia is a common comorbid condition in heart failure patients, and multiple observational studies have demonstrated an independent association between lower hemoglobin and adverse clinical outcomes in such cases [2,3,4]. Chronic anemia is usually accompanied by increased cardiac mass [5,6], but its association with diastolic dysfunction is not well understood. Several published studies support an association between anemia and left ventricular (LV) diastolic dysfunction in patients with diabetes, chronic kidney disease, or cardiovascular disease, but the results are conflicting [7,8,9,10]. There are few previous data on the effects of low hemoglobin levels on LV diastolic function and structure in patients without overt heart disease. The objective of this study was to document changes in the echocardiographic variables of LV structure and function associated with low hemoglobin levels.
METHODS
Study population
Patients were enrolled prospectively from those admitted to Ewha Womans University Mokdong Hospital, from June 2006 to September 2008 with established iron-def iciency anemia without signif icant arrhythmia. Iron-deficiency was defined as having an abnormal value for at least two of three laboratory tests of iron status (erythrocyte protoporphyrin, transferrin saturation, serum ferritin) and iron-deficiency anemia was defined as iron-deficiency plus low hemoglobin (< 12 g/dL) [11]. Exclusion criteria included a history of recurrent anemia episodes, diabetes mellitus, essential hypertension, risk factors (family history of ischemic heart disease, dyslipidemia, or smoking) or established cardiovascular disease. Then, 34 age-and gender-matched healthy individuals with normal hemoglobin concentrations (> 14 g/dL in males and > 12 g/dL in females) were recruited as controls. The controls were free from known diseases, as suggested by history, clinical examination, echocardiography, and basic laboratory investigations. Of the 34 patients with anemia enrolled, 20 were followed and underwent echocardiography after correction of the anemia with oral iron supplements.
This study was approved by the Institutional Review Board of Ewha Womans University Mokdong Hospital. Written informed consent was obtained from all patients.
Echocardiography
Transthoracic echocardiography was performed using a commercially available imaging ultrasound system (Sonos 5500, Hewlett-Packard Co., Palo Alto, CA, USA) with harmonic imaging. All examinations were conducted by experienced sonographers and were analyzed by two experienced observers who were blinded to the clinical data. Quantification of the chamber area was performed basically according to the recommendations of the American Society of Echocardiography [12]. The LV internal diameter, septal thickness, and LV posterior wall thickness (PWT) were measured at end-diastole, defined by the onset of the QRS complex. The LV mass was calculated using the following formula [13]: 0.80 × 1.04 × [(LVEDD + LVST + PWT)3 - (LVEDD)3] + 0.6, where LVEDD is the left ventricular end-diastolic diameter, LVST is the left ventricular septal thickness. The LV mass was indexed to body surface area to obtain the left ventricular mass index (LVMI). The left atrial (LA) diameter was measured by two-dimensional guided M-mode echocardiography, obtained with the parasternal short-axis view at the base of the heart [14]. The LA volume was calculated using the formula for the biplane area-length method [15], and the left atrial volume index (LAVI) was obtained by calculating the LA volume to body surface area ratio. Mitral inf low velocities were obtained by pulsed-wave Doppler in the apical four-chamber view with a 1 to 2 mm sample volume placed at the tips of the mitral valve leaflets. Mitral early (E) and late (A) inflow velocities and the deceleration time (DT) of the E velocity were measured. Using the pulsed-wave tissue Doppler image, early (E') and late (A') diastolic mitral annular velocities were obtained at the septal mitral annulus level in the apical four-chamber view with septal annulus movement aligned with the sample volume line. The ratio of early transmitral inf low to diastolic velocity of the mitral annuls (E/E') was calculated. The left ventricular outflow tract (LVOT) area was calculated from the LVOT diameter, assuming a circular orifice. The stroke volume was calculated as the product of the LVOT area and the time velocity integral of LVOT. The cardiac output was then derived, taking the 1/R-R interval as the heart rate. Cardiac output was indexed to body surface area to obtain cardiac index.
Statistical analysis
Continuous variables are expressed as means ± standard deviations, and categorical variables as numbers and proportions (%). To compare the groups, independent Student t tests were used for continuous variables, and a chi-square test for categorical variables. Parameters measured after correction of anemia were compared with basal values using a paired t test. Correlations between variables were assessed with the Pearson correlation test. A p value of < 0.05 was considered to indicate statistical significance.
RESULTS
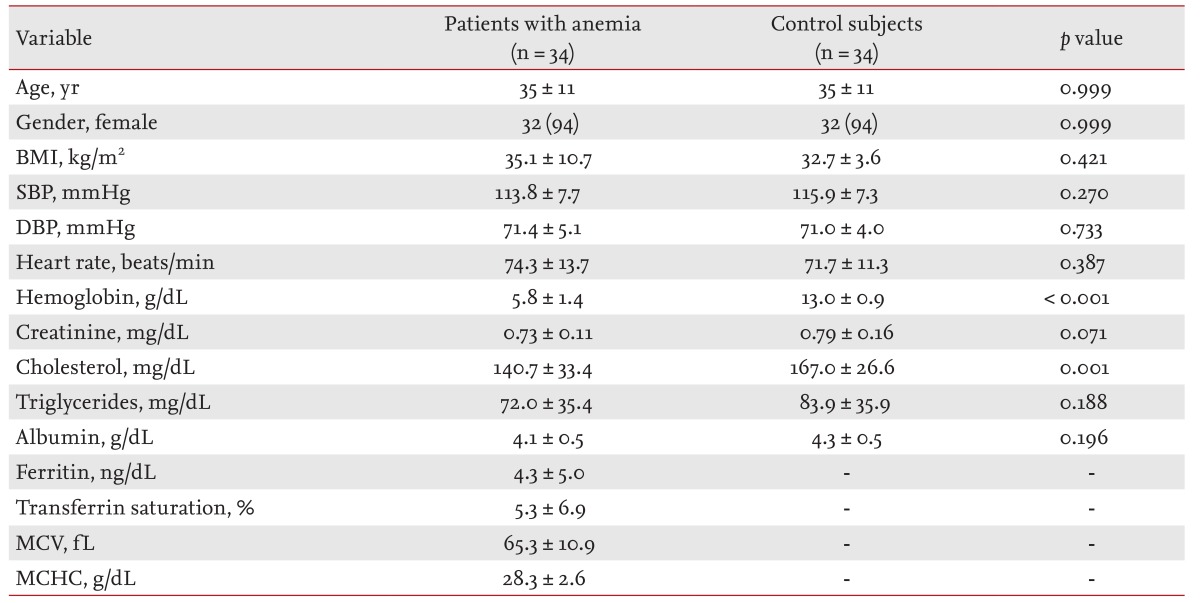
Baseline characteristics of anemic patients and control subjects are summarized in Table 1. There was no significant difference between the groups in age, gender, body mass index, systolic or diastolic blood pressure, heart rate, serum creatinine, serum triglycerides, or serum albumin. Serum cholesterol and mean hemoglobin levels were lower in the anemia group than the controls. The mean values of ferritin, transferrin saturation, mean corpuscular volume and mean corpuscular hemoglobin concentration in patients with anemia were lower than previous reference values.
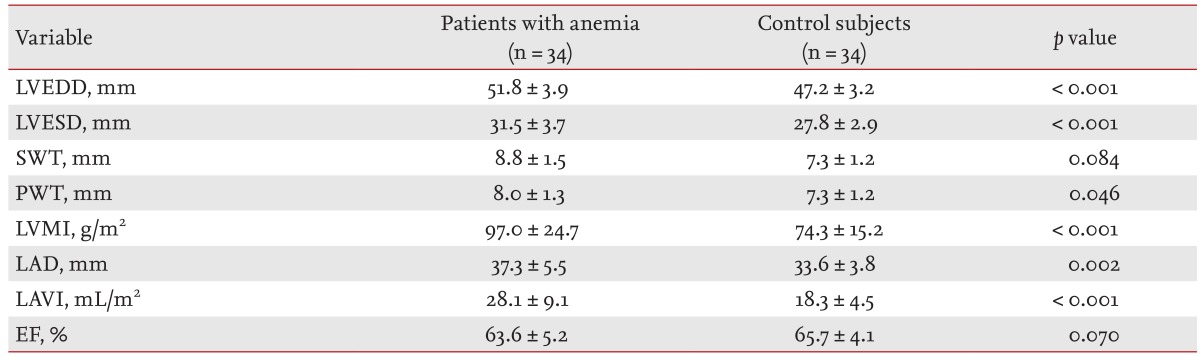
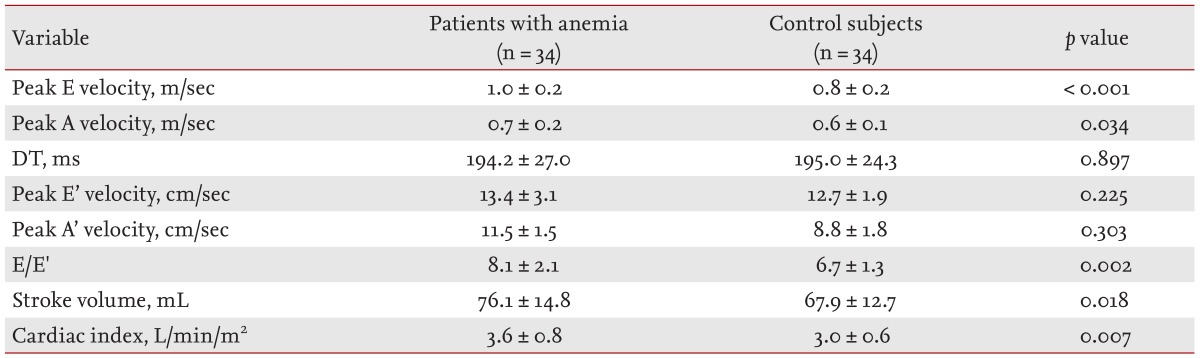
The two-dimensional echocardiographic measurements are shown in Table 2. The LVEDD and left ventricular end-systolic diameter (LVESD), LV PWT, and LVMI were significantly elevated in the anemia group. The LV ejection fraction did not differ significantly between the groups. LAVI was increased in patients with anemia. Doppler measurements are shown in Table 3. The peak E velocity, peak A velocity, E/E' ratio, stroke volume, and cardiac index were elevated significantly in the anemia group. There was no significant difference in DT, peak E' velocity, or peak A' velocity.
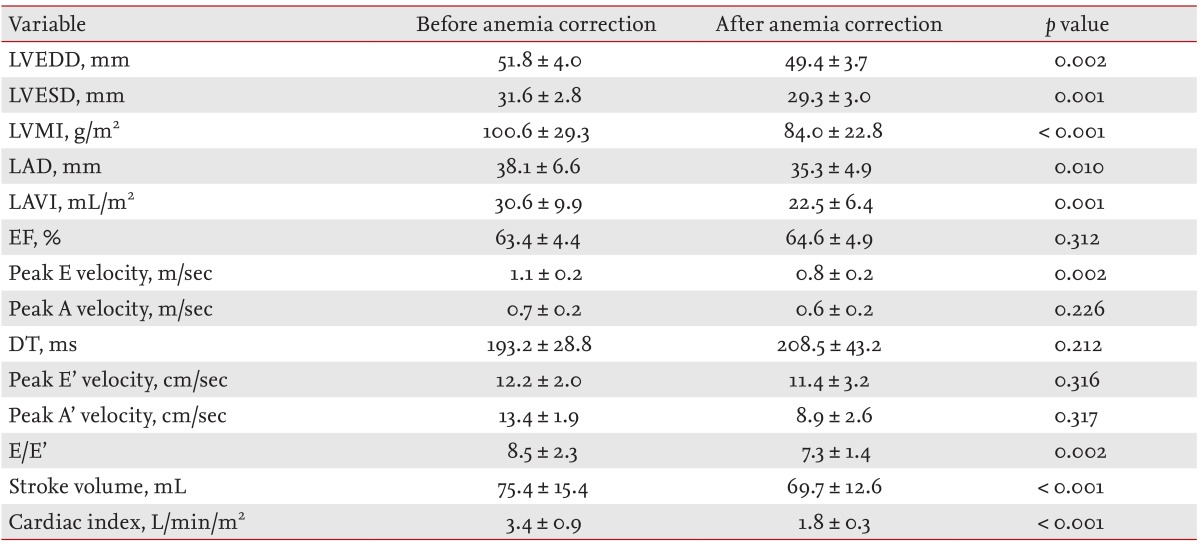
Of the 34 patients with anemia, 20 underwent follow-up echocardiography after correction of the anemia with iron replacement therapy and transfusion. The mean time between baseline and follow-up was 5.2 ± 3.9 months. Clinical features and laboratory findings of the patients with anemia after iron replacement therapy are shown in Table 4. There was no significant change in body mass index, systolic blood pressure, or diastolic blood pressure. The heart rate was significantly lower on follow-up, and mean hemoglobin was increased significantly compared with the baseline level (5.8 ± 1.3 g/dL vs. 12.5 ± 1.4 g/dL; p < 0.001).

Clinical features and laboratory findings of anemic patients (n = 20) before and after anemia correction
Echocardiographic variables of anemic patients on follow-up are shown in Table 5. The LVESD, LVEDD, and LVMI decreased significantly, as was the LAVI. The ejection fraction did not change. In the Doppler echocardiographic studies, peak E velocity, E/E', stroke volume and cardiac index all decreased significantly and peak A velocity, DT, peak E' velocity, and peak A' velocity were unchanged. A correlation analysis between hemoglobin and echocardiographic variable changes after correction of anemia showed a significant negative correlation between percent change in hemoglobin and change in E/E' and between percent change in hemoglobin and percent change in E/E' (p = 0.003 and p = 0.012, respectively) (Fig. 1). However, there was no significant correlation between change in hemoglobin and LVEDD, LVESD, LVMI, LAVI, or cardiac index.

Scatter plot showing the correlation (A) between the percent change in hemoglobin level and change in the ratio of early transmitral inflow to diastolic velocity of the mitral annuls (E/E', and (B) between the percent change of hemoglobin level and the percent change of E/E' in the anemic patients after correction of anemia.
DISCUSSION
Anemia is highly prevalent in heart failure patients and is strongly associated with systolic dysfunction [16].
Treatment of anemia improved exercise capacity and symptoms in symptomatic heart failure patients [17,18,19]. Anemia is also a known risk factor for cardiovascular disease, even in the low-risk general population [20]. However, it is unclear whether the correction of anemia benefits cardiac function or geometry. In the present study, we observed a significant improvement in LV geometry including LV mass and cavity size and a decrease in LV filling pressure in patients with iron-deficiency anemia without overt cardiovascular disease after correction of the anemia.
Most studies have been based on indices of systolic function and LV mass, and the association of anemia with LV diastolic function has been conflicting. Bahl et al. [9] did not observe any consistent abnormality in diastolic function in anemic conditions. Srivastava et al. [7] found that diabetic patients with anemia showed increased LA area, E/E' ratio, and E-to-propagation velocity (E/Vp) ratio, compared with diabetic patients without anemia, but any causal relationship between anemia and diastolic dysfunction was uncertain. Nair et al. [8] reported that coronary artery disease with moderate and severe anemia was associated with diastolic dysfunction, assessed by the mitral inf low pattern.
We evaluated LV diastolic filling pressure using the LAVI and E/E' ratio. The E/E' ratio is used as an initial estimate of the LV filling pressure, particularly in patients with preserved systolic function. The LA volume can be viewed as a morphological expression of LV diastolic dysfunction, because the exposure of the LA to increased filling pressure results in its remodeling, which is reflected in the LA volume [21,22]. We found that anemia was strongly associated with LV filling pressure, as assessed by the E/E' ratio and the LAVI. These functional and hemodynamic changes in the LV associated with anemia were improved by appropriate correction of the low hemoglobin level. Thus, our results suggest that increased LV filling pressure and enlarged LA may be important mediators in the pathway from anemia to heart failure and the changes of LV geometry and filling pressure can be recovered, at least partly, by appropriate correction of hemoglobin levels.
Several mechanisms may explain the association between anemia and raised LV filling pressure. Adaptation to an anemic state involves augmentation of the cardiac index and stroke volume [23]. This overall increase in sympathetic and inotropic activity places additional stress on the myocardium, perhaps leading to remodeling of the myocytes and vasculature [9]. Additionally, studies on the coronary blood flow in anemic patients have shown a decrease in myocardial oxygen consumption despite an increase in myocardial workload and oxygen extraction [9]. As myocardial ischemia, as exemplified by coronary artery disease, is a well-established cause of diastolic dysfunction and increased LV filling pressure [24], the inf luence of hypoxia on patients with anemia may have a similar effect. Interestingly, DT and peak E' velocity, which are parameters of LV relaxation [25], did not differ between anemic patients and the control group and did not change after correction of anemia in our study. Thus, we suggest that increased LV filling pressure, resulting from the hyperdynamic status, may be more important than LV relaxation in causing the increase in E/E' and LV structural changes in anemic patients.
Our results also are consistent with previous reports in which patients with anemia tended to be in a hyperdynamic state [9,23]. These patients had significantly higher stroke volumes and cardiac indices. Similarly, the LV mass and diameter were also increased significantly in these patients. Various factors have been implicated to explain this hyperdynamic state. Increased LVEDD suggested a role for the Frank-Starling mechanism in the hyperdynamic state of anemia [9]. Moreover, the presence of a significantly reduced systemic vascular resistance may contribute to the hyperdynamic state in such patients [9]. Classically, anemia has been associated with eccentric LV growth, characterized by a proportional increase in the LV diameter and wall thickness [6,26]. Our results suggest that the pattern of LV structural change associated with anemia involves an increase in both wall thickness and in LV cavity size.
Hemoglobin level was associated with structural changes in LA and LV, as well as the change in E/E', which reflects LV filling pressure and LA pressure, but the values were all within normal ranges during the follow-up period. However, there were significant serial changes during follow-up, even though the follow-up duration was not long in this study. Thus, a further long-term effect of anemia on LV may be more extensive and this may be worth considering when managing heart failure patients with anemia. After correcting the anemia, only changes in the E/E' ratio were negatively related to changes in the hemoglobin level. This indicates that the LV filling pressure, assessed by the E/E' ratio, may be the best method of predicting the current status of the LV, which is influenced by hemoglobin changes.
Limitations
We believe that our study helps clarify the role of anemia in cardiac function and structure, but several limitations should be mentioned. First, our population was composed largely of young females, and our results may not be pertinent to males or to other age groups. Second, the number of patients who completed echocardiographic follow-up in the study was relatively small. Thus, there were limitations in terms of performing multivariate analyses. Third, among the baseline characteristics, cholesterol levels showed significant differences between the anemic patients and controls. Additionally, heart rate and cholesterol level changed significantly in the studied population during the follow-up period. These factors may also have affected the results. Thus, further larger-scaled studies are warranted to reduce the effects of these confounding factors. Fourth, our population was composed totally of iron-def iciency anemia, and it is unclear whether our findings are applicable to patients with other causes of anemia. Fifth, most of the patients in our population demonstrated severe anemia, with hemoglobin as low as 3.3 g/dL, so we could not leave them without treatment for ethical reasons. Thus, there were no controls with uncorrected low hemoglobin levels on follow-up.
In conclusion, an association between anemia and LV structure and filling pressure was found in this study. The LVEDD, LVESD, LVMI, LAVI, and E/E' ratio changed significantly after appropriate correction of the low hemoglobin levels in anemia patients without traditional cardiovascular risk factors or cardiovascular disease. These findings support the notion that the correction of anemia might improve LV structure and function.
KEY MESSAGE
1. Low hemoglobin level was associated with larger cardiac chambers, increased left ventricle (LV) mass and higher LV filling pressure.
Notes
No potential conflict of interest relevant to this article was reported.