Predictors and clinical outcomes of persistent methicillin-resistant Staphylococcus aureus bacteremia: a prospective observational study
Article information
Abstract
Background/Aims
The high mortality attributable to persistent methicillin-resistant Staphylococcus aureus (MRSA) bacteremia in spite of glycopeptide treatment has heightened the need for early detection and intervention with alternative agents. The purpose of this study was to determine the clinical characteristics of and risk factors for persistent MRSA bacteremia.
Methods
All first episodes of significant MRSA bacteremia at a 710-bed academic medical center from November 2009 through August 2010 were recorded. Blood cultures were conducted at 3 days and every 2 to 3 days thereafter until clearance. Clinical characteristics and outcomes were compared between persistent MRSA bacteremia (≥ 7 days) and nonpersistent MRSA bacteremia (≤ 3 days).
Results
Of 79 patients with MRSA bacteremia during the study period, 31 (39.2%) had persistent MRSA bacteremia. The persistent MRSA bacteremia group had significantly higher 30-day mortality than the nonpersistent MRSA bacteremia group (58.1% vs. 16.7%, p < 0.001). Multivariate analysis indicated that metastatic infection at presentation (odds ratio [OR], 14.57; 95% confidence interval [CI], 3.52 to 60.34; p < 0.001) and delayed catheter removal in catheter-related infection (OR, 3.80; 95% CI, 1.04 to 13.88; p = 0.004) were independent predictors of persistent MRSA bacteremia. Patients with a time to blood culture positivity (TTP) of < 11.8 hours were at increased risk of persistent MRSA bacteremia (29.0% vs. 8.3%, p = 0.029).
Conclusions
High mortality in patients with persistent MRSA bacteremia was noted. Early detection of metastatic infection and early removal of infected intravascular catheters should be considered to reduce the risk of persistent MRSA bacteremia. Further studies are needed to evaluate the role of TTP for predicting persistent MRSA bacteremia.
INTRODUCTION
Methicillin-resistant Staphylococcus aureus (MRSA) is a significant cause of healthcare-associated morbidity and mortality in most countries [1-3]. The prevalence of MRSA infections and glycopeptide treatment of MRSA are increasing. However, treatment failure of MRSA bacteremia has been described, despite the organism being fully susceptible to glycopeptides [4-6].
Recent studies have focused on persistent MRSA bacteremia and its poor clinical outcome. A recent report identified the retention of implicated medical devices, MRSA infection in at least two sites, and a vancomycin minimal inhibitory concentration (MIC) of 2 µg/mL as independent risk factors for persistent MRSA bacteremia [7]. Endocarditis, septic shock, complicated bacteremia, decreased vancomycin susceptibility, heteroresistance, agr dysfunction, and low-level in vitro resistance to thrombin-induced platelet microbicidal protein were also implicated as independent risk factors for persistent MRSA bacteremia in other studies [8-10]. Additionally, one study showed that metastatic infection, congestive heart failure, and elevated vancomycin MICs for subsequent MRSA isolates were independent predictors of 30-day mortality in persistent MRSA bacteremia [11]. Although MRSA bacteremia has received great attention in the medical literature, persistent MRSA bacteremia remains a challenging clinical problem.
In Korea, MRSA strains account for about 70% to 80% of nosocomial S. aureus infections [12] and the impact of the increasing MRSA bacteremia frequency is magnified by the poor prognosis associated with this serious infection [13]. In this study, we assessed the clinical characteristics and predictors of persistent MRSA bacteremia with glycopeptide treatment, and also investigated the relationship between time to blood culture positivity (TTP) and persistence in MRSA bacteremia.
METHODS
Study design and patient population
All patients aged 18 years or older with positive blood MRSA cultures at a 710-bed hospital in the Republic of Korea during the period November 2009 through August 2010 were included in this prospective study. Only the first episode of MRSA bacteremia for each patient was included in the analysis. Patients were excluded if they received glycopeptide treatment within the 48 hours prior to collection. The following data were recorded for all patients: demographic characteristics, site(s) of infection at presentation, TTP, empirical glycopeptide use, time to initiation of glycopeptide treatment, catheter removal within 24 hours after the onset of MRSA bacteremia, vancomycin MIC of the strain, and 30-day crude mortality. Pitt bacteremia scores were calculated to determine illness severity. Therapeutic drug monitoring of vancomycin was routinely conducted before the fourth dose. The vancomycin dosage was adjusted to obtain a trough concentration of > 15 µg/mL. Blood cultures were conducted 3 days after the initiation of glycopeptide treatment and every 2 to 3 days thereafter until clearance. Transthoracic echocardiography (TTE) was performed in all patients with persistent MRSA bacteremia (≥ 7 days). The patients' physicians determined the patient management and treatment regimens. Vancomycin or teicoplanin use was controlled under the hospital antibiotic restriction program. This study was approved by our Institutional Review Board, and informed consent was waived due to the observational nature of the study.
Definitions
Clinically significant bacteremia was defined as at least one positive blood culture, together with clinical features consistent with systemic inflammatory response syndrome. Persistent MRSA bacteremia was defined as bacteremia persisting for ≥ 7 days after initiation of glycopeptide treatment. Nonpersistent MRSA bacteremia was defined as bacteremia persisting for ≤ 3 days after initiation of glycopeptide treatment. Catheter-related blood stream infection (CRI) was defined as growth of > 15 MRSA colony-forming units from the catheter tip in semiquantitative culture or growth of MRSA from a blood sample drawn from a catheter hub at least 2 hours before MRSA was obtained from a peripheral vein blood sample [14]. Infective endocarditis was defined according to the modified Duke criteria [15]. Metastatic infection was defined as microbiological or radiological evidence of MRSA infection caused by hematogenous seeding. Empirical glycopeptide use was defined as the administration of glycopeptides within 24 hours after the acquisition of blood culture samples. Early and delayed catheter removal were defined as catheter removal within 24 hours of the onset of MRSA bacteremia and more than 24 hours after the onset of MRSA bacteremia, respectively. The primary outcome was all-cause mortality within 30 days after blood culture collection.
Microbiological methods
The BacT ALERT 3D culture system (bioMérieux Inc., Hazelwood, MO, USA) for blood cultures was used. Blood culture bottles were placed in the blood culture system at any time of the day. TTP was defined as the time between incubation onset and detection of growth using an automated blood culture system. MRSA identification and vancomycin MIC tests were performed using VITEK II standard identification and susceptibility cards (bioMérieux Inc.), in accordance with the manufacturer's instructions.
Statistical analysis
Categorical variables were calculated as percentages and continuous variables were summarized as means with standard deviations or medians with interquartile ranges. Clinical characteristics and outcomes were compared between persistent MRSA bacteremia and nonpersistent MRSA bacteremia using Fisher exact and Mann-Whitney U tests. Stepwise logistic regression analysis was used to control for the effects of confounding variables when identifying predictors of persistent MRSA bacteremia. Variables with a p < 0.05 in the univariate analysis were included in the subsequent multivariate analysis. Forward stepwise variable elimination was then performed. Receiver operator characteristic curves were plotted for TTP to predict persistent MRSA bacteremia and the area under the curve (AUC) was calculated. The Kaplan-Meier method was used for survival analysis. The p values < 0.05 were considered to indicate statistical significance. All analyses were performed using the SPSS software version 18.0 for Windows (PASW, IBM Co., Armonk, NY, USA).
RESULTS
A total of 79 patients with MRSA bacteremia were identified during the study period. Of these patients, 12 were excluded from the analysis for the following reasons: death within 1 day of culture results (three patients), glycopeptide treatment within the 48 hours prior to collection (five patients), and bacteremia of intermediate duration (4 to 6 days, four patients). Of the 67 patients analyzed, 31 (46.3%) had persistent MRSA bacteremia.
Baseline characteristics for persistent and nonpersistent MRSA bacteremia
The baseline demographic and clinical characteristics of the persistent MRSA bacteremia group were compared with those of the nonpersistent MRSA bacteremia group (Table 1). Underlying diseases did not differ between the two groups, with the exception of cardiovascular disease (45.2% vs. 16.7%; p = 0.011). In terms of illness severity, Pitt bacteremia scores were higher in the persistent MRSA bacteremia group (median 2 vs. 1, p = 0.025); however, Charlson weighted index of comorbidity did not differ between the two groups (median 3.0 vs. 2.5, p = 0.321). With respect to the infection site(s) at presentation, CRI (51.6% vs. 22.2%, p = 0.012) and metastatic infection at presentation (58.1% vs. 8.3%, p < 0.001) were more common in the persistent MRSA bacteremia group. With respect to TTP, the AUC was 0.647 (95% confidence interval [CI], 0.520 to 0.759) and the diagnostic sensitivity was 91.7% (95% CI, 77.5 to 98.2). The specificity for predicting persistent MRSA bacteremia was 32.3% (95% CI, 16.7 to 51.4) with glycopeptide therapy and a TTP value of 11.8 hours (Fig. 1). Patients with low TTP values (< 11.8 hours) were more common in the persistent MRSA bacteremia group (29.0% vs. 8.3%, p = 0.029).
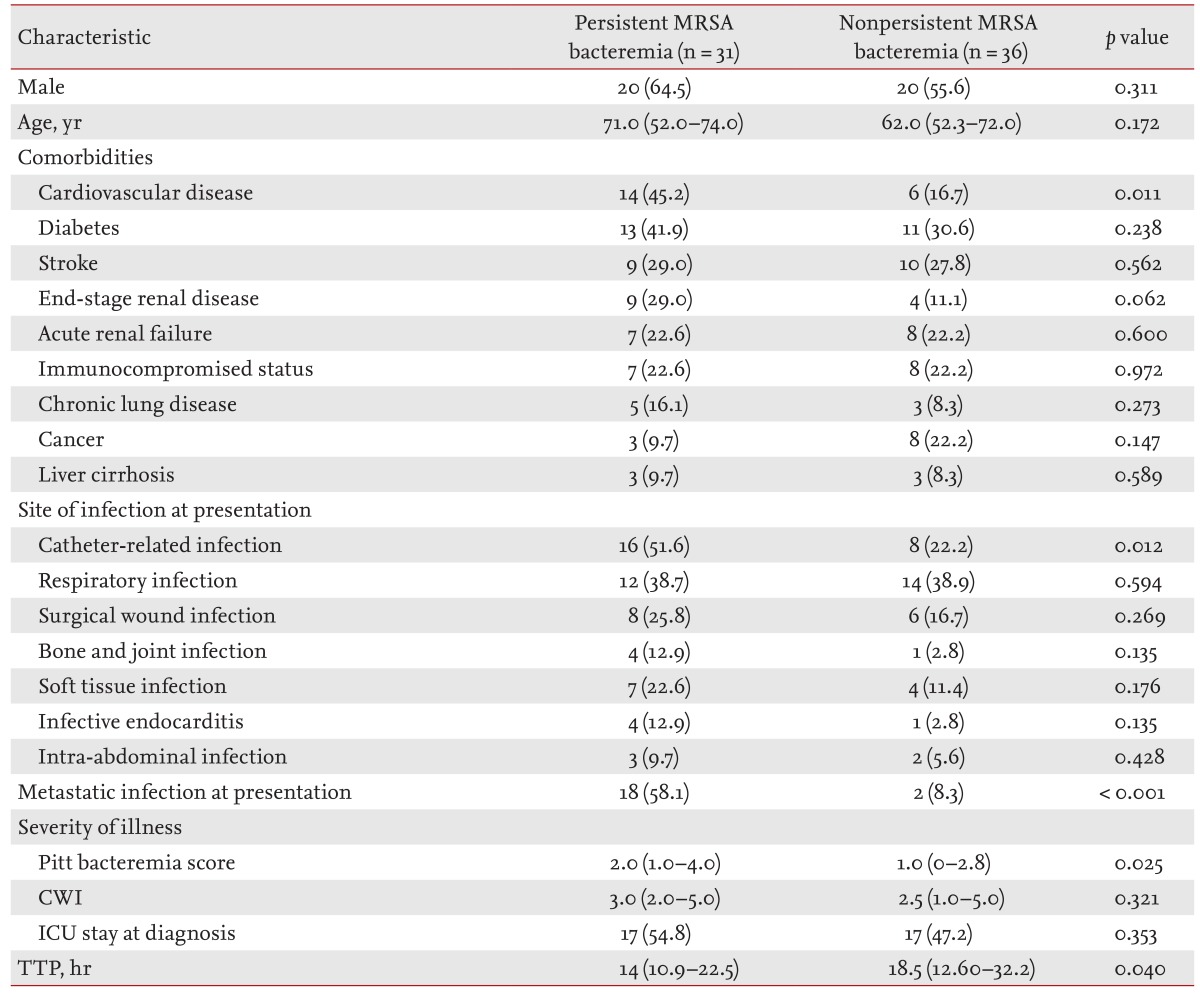
Clinical characteristics and outcomes of persistent and nonpersistent methicillin-resistant Staphylococcus aureus bacteremia
Predictors of persistent MRSA bacteremia
A multivariate analysis of potential risk factors associated with persistent MRSA bacteremia was conducted. Variables with a p < 0.05 in the univariate analysis (metastatic infection at presentation, CRI, cardiovascular disease, TTP < 11.8 hours, and Pitt bacteremia score) were included in the subsequent multivariate analysis. Stepwise forward logistic regression analysis revealed that delayed catheter removal in CRI (odds ratio [OR], 3.80; 95% CI, 1.04 to 13.88; p = 0.044) and metastatic infection at presentation (OR, 14.57; 95% CI, 3.52 to 60.34; p < 0.001) were independent predictors of persistent MRSA bacteremia.
Clinical management and outcomes in persistent and nonpersistent MRSA bacteremia
Clinical management and outcomes in persistent MRSA bacteremia are summarized in Table 2. Empirical glycopeptide use and time to initiation of glycopeptide treatment did not differ between the two groups. The initial use of vancomycin or teicoplanin was also similar in the two groups (77.4% vs. 77.1%, p = 0.606).

Clinical management and outcomes of persistent and nonpersistent methicillin-resistant Staphylococcus aureus bacteremia
Retention of central lines occurred in four of the 24 patients with catheter-associated infections. Catheters were removed within 24 hours of collection of the first positive culture in 16 patients. There was no difference between the persistent and nonpersistent MRSA bacteremia groups in retention of central lines and delayed catheter removal (12.5% vs. 25.0%, p = 0.407 and 25.0% vs. 50.0%, p = 0.221, respectively).
Subgroup analysis was performed in patients treated with vancomycin (n = 51). Vancomycin MICs of 2 µg/mL (25.8% vs. 27.8%, p = 0.539), serum vancomycin trough concentrations of > 15 µg/mL (62.5% vs. 55.6%, p = 0.722), and time until a vancomycin trough of > 15 µg/mL (119 hours vs. 87 hours, p = 0.126) did not differ between the two groups.
Thirty-day cumulative survival was 41.9% for patients with persistent MRSA bacteremia and 83.3% for patients with nonpersistent MRSA bacteremia (Fig. 2). The difference was statistically significant (p < 0.001, log-rank test).

Thirty-day Kaplan-Meier survival curves for patients with methicillin-resistant Staphylococcus aureus (MRSA) bacteremia. The cumulative survival rate of patients with persistent MRSA bacteremia was significantly lower than that of patients with nonpersistent MRSA bacteremia (p < 0.001, log-rank test).
DISCUSSION
This study found that patients with persistent MRSA bacteremia had significantly higher mortality, and that delayed catheter removal in CRI and metastatic infection at presentation were independent predictors of persistent MRSA bacteremia. The incidence of persistent MRSA bacteremia with glycopeptide treatment in this study (31/79, 39.2%) was similar to that in a previous study conducted in Korea (31/96, 32.3%) [7]. However, the 30-day mortality rate (58.1%) was higher (45.2%) [7]. These rates are higher than those reported for other countries, in which mortality rates in patients with persistent MRSA bacteremia ranged from 15.8% to 25.6% [8,9]. One possible explanation for these findings is that the time to initiation of glycopeptide therapy after the onset of MRSA bacteremia was longer in our study than that in a previous report (34.8 hours vs. 19.5 hours) [8]. Other possible explanations could relate to the incidence of endocarditis and the proportion of vancomycin MICs of 2 µg/mL in our study. The incidence of endocarditis in patients with persistent bacteremia in our study (4/31, 13%) was lower than incidence rates in previous reports [8,11]. Our results may, in fact, underestimate the actual incidence as we used TTE to detect endocarditis. In addition, the incidence of vancomycin MICs of 2 µg/mL in patients with persistent MRSA (33.3%) was higher in our study than in a previous study (26.4%) [11]. Previous studies reported that higher vancomycin MICs resulted in worse clinical outcomes including increased mortality, clinical failure, and vancomycin treatment failure [16-18].
CRIs were more common in the persistent MRSA bacteremia group. However, delayed catheter removal in patients with CRI was an important confounder that might alter the risk of persistence in MRSA bacteremia. We conclude that delayed catheter removal in CRI and metastatic infection at presentation were independent predictors of persistent MRSA bacteremia after properly controlling for confounders in the statistical analysis. These findings are consistent with a previous report that delayed catheter removal is a risk factor for hematogenous complications in patients with MRSA bacteremia [19].
Our results also highlighted the clinical significance of TTP in persistent MRSA bacteremia. Persistent MRSA bacteremia was more frequent in patients with a TTP of < 11.8 hours. Khatib et al. [20] reported that a TTP of < 14 hours was an independent predictor of an endovascular source of infection, persistent bacteremia, metastatic infection, and attributable mortality. It has also been reported that there is a correlation between a higher bacterial load and low TTP values [21,22]. Therefore, TTP has been suggested as a surrogate marker for quantification of bacterial load and for adverse outcomes in bacteremia [22,23]. LaPlante and Rybak [24] reported that the AUC/MIC ratio was reduced by 50% for both MSSA and MRSA isolates when vancomycin levels were evaluated at high bacterial inocula. In this study, the low TTP values in patients with persistent bacteremia may have been due to a higher bacterial load and may have corresponded to a subsequently reduced AUC/MIC ratio. This may have resulted in higher mortality, because the AUC/MIC ratio is considered to be the most important pharmacodynamic parameter associated with positive clinical outcomes [25].
Previous studies found that MRSA bacteremia with higher vancomycin MIC values (≥ 2 µg/mL) was an independent predictor of persistence [7,8]. One study showed that there was no correlation between MIC and duration of positive blood cultures in a pediatric population [26]. Some studies showed that there might be geographical variation in the usefulness of the vancomycin MIC as a predictor of outcome [27]. In the current study, the incidence of vancomycin MICs of 2 µg/mL did not significantly differ between the persistent and nonpersistent bacteremia groups. Interestingly, three patients in the persistent MRSA bacteremia group showed elevation of vancomycin MICs for serial MRSA isolates and TTPs of less than 12 hours (data not shown). These results imply that low-grade vancomycin resistance could develop during the treatment of persistent MRSA bacteremia. These findings are consistent with a previous study in which elevated vancomycin MICs were found in two (18.2%) of 11 pairs of MRSA isolates from patients with persistent MRSA bacteremia [28]. Lin et al. [11] reported a significant association between treatment outcome and vancomycin MIC elevation for serial MRSA isolates during persistent bacteremia.
Our study had some limitations. First, few cases were evaluated, which limited statistical the power; however, despite its relatively small size, the study population was adequate to identify risk factors for persistent MRSA bacteremia. Second, other virulence and genetic factors implicated in invasiveness, disease severity, and persistent bacteremia were not evaluated. Third, vancomycin MICs were determined using an automated system. The E-test method has been found to provide better prediction of treatment outcomes in MRSA infections than automated or broth microdilution methods [29]. Fourth, we did not determine the prevalence of vancomycin heteroresistance. Because vancomycin heteroresistance has been reported to be associated with persistent bacteremia [30], assessment of possible heteroresistance is necessary as approximately one-quarter of the isolates had high vancomycin MICs (2 µg/mL). Fifth, TTE was conducted in lieu of transesophageal echocardiography. Therefore, endocarditis may have been underestimated. Finally, we accessed 30-day all-cause mortality instead of infection-related mortality. Although attributable mortality is important, especially considering the proportion of older patients in our study, all-cause mortality is frequently used in S. aureus outcome studies [17,26,31,32]. Nonetheless, we performed blood culture at regular intervals and included all patients who received other MRSA-active agents, which may have helped to avoid selection bias. The prospective design is another strength of this study.
In conclusion, the high mortality attributable to persistent MRSA bacteremia was noted even in those who had undergone glycopeptide treatment. Factors independently associated with persistent MRSA bacteremia included delayed removal of an infected intravascular catheter and disseminated infection at presentation. Early catheter removal in CRI and evaluation of disseminated infections should be considered to reduce the risk of persistent MRSA bacteremia. Further studies should evaluate the role of low TTP values as a predictor in persistent MRSA bacteremia.
KEY MESSAGE
1. The high mortality attributable to persistent methicillin-resistant Staphylococcus aureus (MRSA) bacteremia was noted even in those who had undergone glycopeptide treatment.
2. Factors independently associated with persistent MRSA bacteremia included delayed removal of an infected intravascular catheter and disseminated infection at presentation.
3. Early detection of metastatic infection and early removal of an infected intravascular catheter should be taken into consideration for reducing the risk of persistent MRSA bacteremia.
Notes
This study was presented, in part, at the 21st European Congress of Clinical Microbiology and Infectious Diseases and the 27th International Congress of Chemotherapy, Milan, Italy, 7 to 10 May 2011.
No potential conflict of interest relevant to this article is reported.
