Meta-analysis of randomized controlled trials of bosentan for treatment of pulmonary arterial hypertension
Article information
Abstract
Background/Aims
We assessed the efficacy and safety of bosentan in patients with pulmonary arterial hypertension (PAH).
Methods
We surveyed randomized controlled trials (RCTs) of the efficacy and safety of bosentan in patients with PAH using MEDLINE, EMBASE, the Cochrane Controlled Trials Register, and manual searches. Meta-analysis of RCTs was performed to determine treatment efficacy and safety outcomes. Results are presented as odds ratios (ORs) or weighted mean differences (WMDs).
Results
Meta-analysis of seven RCTs including a total of 410 patients and 296 controls revealed that the 6-minute work distance was significantly higher in the bosentan group than in the placebo group (WMD, 46.19; 95% confidence interval [CI], 21.20 to 71.19; p = 2.9 × 10-5). Compared with the placebo, bosentan significantly reduced the mean pulmonary arterial pressure in patients with PAH (WMD, -6.026; 95% CI, -8.785 to -3.268, p = 1.8 × 10-6). The bosentan therapy group worsened less clinically than the placebo group (OR, 0.252; 95% CI, 0.140 to 0.454; p = 4.6 × 10-7). The incidence of serious adverse events did not differ between the bosentan and placebo groups (OR, 0.948; 95% CI, 0.556 to 1.614; p = 0.843). However, the results of the abnormal liver function test (LFT) were significantly higher in the bosentan group than in the placebo group (OR, 2.312; 95% CI, 1.020 to 5.241; p = 0.045).
Conclusions
This meta-analysis shows that bosentan can treat PAH effectively. However, bosentan increased the incidence of abnormal LFT results compared with the placebo.
INTRODUCTION
Pulmonary arterial hypertension (PAH) is a devastating, progressive disease characterized by increases in pulmonary vascular resistance due to vasoconstriction and remodeling which lead to increased pulmonary arterial pressure (PAP), right ventricular failure, and death [1].
Endothelial dysfunction plays a key role in PAH pathogenesis, inducing chronically impaired production of vasodilator and antiproliferative agents such as nitric oxide and prostacyclin, along with causing overexpression of vasoconstrictor and promitotic molecules such as entothelin-1 (ET-1) [2]. ET-1 is a 21-amino acid peptide that exerts vasoconstrictor and mitogenic effects by binding to two distinct receptor isoforms in pulmonary vascular smooth muscle cells: endothelin receptor type A (ETA) and B (ETB) [3].
Bosentan is an orally active dual endothelin receptor antagonist that has been demonstrated to improve exercise capacity, hemodynamics, and slow clinical worsening in clinical trials [4]. However, the clinical relevance of these effects is unknown because of the short duration and small sizes of the individual studies [4-10].
Meta-analysis is a statistical procedure that combines the results of several studies to produce a single estimate of a major effect with enhanced precision [11]. The major advantage of meta-analysis is that it increases sample size, which possibly reduces the probability that random error will produce false-positive or false-negative associations [12-14]. Thus, the aim of the present study was to evaluate the efficacy and safety of bosentan in PAH patients via meta-analysis.
METHODS
Identification of eligible studies and data extraction
We performed an exhaustive search of studies that examined the efficacy and safety of bosentan in patients with PAH. The literature was searched using MEDLINE, EMBASE, and the Cochrane Controlled Trials Register to identify available articles (up to November 2012). The following key words and subject terms were used in the searches: bosentan, pulmonary arterial hypertension, and pulmonary hypertension. All references in the studies were reviewed to identify additional works not indexed by the electronic databases. Randomized controlled trials (RCTs) were included if they met the following criteria: 1) the study compared bosentan with a placebo for PAH, 2) the study provided endpoints for the clinical efficacy and safety of bosentan, and 3) the study included patients diagnosed with PAH based on clinical classification. The exclusion criteria were as follows: 1) the study compared bosentan with drugs such as phosphodiesterase type 5 inhibitors (sildenafil) or prostanoids (iloprost) for PAH, 2) the study used combination therapy with sildenafil or iloprost for PAH, and 3) the study included duplicated data or did not contain adequate data for inclusion.
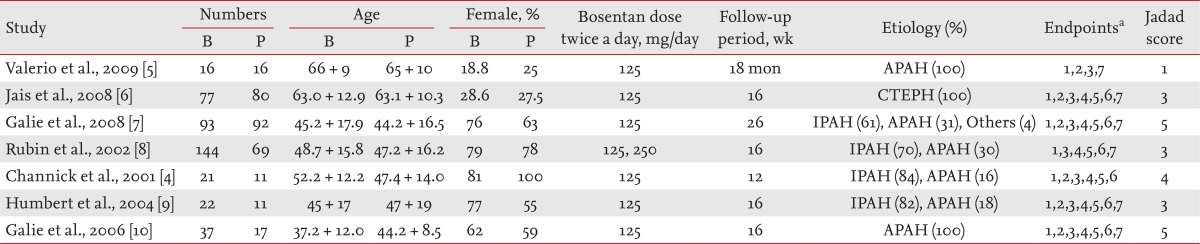
Efficacy outcomes were as follows: 1) 6-minute work distance (6-MWD), 2) mean pulmonary arterial pressure (mPAP), 3) clinical worsening, and 4) New York Heart Association/World Health Organization (WHO) functional class amelioration. Clinical worsening refers to the need for hospitalization due to the emergence of right heart dysfunction or progressive increase of PAP and the necessity of withdrawal from the trial due to the need to alter medication, or the occurrence of interatrial fistulas, lung transplantation, or death because clinical symptoms were not alleviated or were aggravated [15]. The safety outcomes were serious adverse events (SAE) that were considered to be related to the medication, an abnormal liver function test (LFT), and mortality due to any cause. The following information was extracted from each study: initial author, year of publication, bosentan dose, length of follow-up, and efficacy and safety outcomes. We quantified the methodological qualities of the studies using Jadad scores [16]. These assessments were based on: 1) whether the randomization method was appropriate, 2) whether double blindness was mentioned in the trial and the trial was appropriately performed, and 3) whether the number of patients that withdrew or dropped out, and the reasons for this, were clearly stated. Regarding the meta-analysis methods and results, data extraction and quality control was performed by two independent reviewers (G.G.S. and Y.H.L.). Discrepancies between the reviewers were resolved by consensus.
Evaluation of publication bias
Funnel plots are normally used to detect publication bias. However, since funnel plots require a range of studies of different sizes and subjective judgments, we evaluated publication bias using Egger's linear regression test [17], which measures funnel plot asymmetry using a natural logarithm scale.
Evaluation of statistical associations
We calculated odds ratios (OR) for dichotomous data, weighted mean differences (WMD) for continuous data, and corresponding 95% confidence intervals (CIs). We assessed within- and between-study variation and heterogeneities using Cochran Q-statistics [18]. The heterogeneity test was used to assess the null hypothesis that all studies were evaluating the same effect. When a significant Q-statistic (p < 0.10) indicated heterogeneity across studies, the random-effect model was used for the meta-analysis, and when it did not, the fixed-effect model was used. The fixed effect model assumes that all studies estimate the same underlying effect and considers only within-study variation. We quantified the effect of heterogeneity using I2 = 100% × (Q - df) / Q [19], where I2 measures the degree of inconsistency between studies and determines whether the percentage total variation across studies is due to heterogeneity rather than to chance. I2 ranges between 0% and 100%; I2 values of 25%, 50%, or 75% are referred to as low, moderate, and high estimates, respectively. Statistical analyses were performed using the Comprehensive Meta-Analysis software (Biostat, Englewood, NJ, USA).
RESULTS
Studies included in the meta-analysis
One hundred and twenty-five studies were identified by electronic or manual searches and 10 were selected for full-text review based on the title/abstract [4-10,20-22]. However, three of the 10 were excluded; two contained duplicate data [20,21] and one did not contain adequate data for inclusion [22]. Thus, seven studies met the inclusion criteria (Fig. 1) [4-13]. These seven studies involved a total of 410 patients and 296 controls. The characteristic features of the studies included in the meta-analysis are given in Table 1. In all studies, patients received bosentan daily, except one study, in which they received both bosentan and epoprostenol [7]. The minimum bosentan dose was 125 mg twice a day in all studies, but in one study patients received doses of 125 mg and 250 mg twice a day. Follow-up periods ranged from 12 to 26 weeks in six studies, but one study had a long follow-up period (18 months), and the median Jadad score was 3 (range, 1 to 5) (Table 1). Seven RCTs evaluated 6-MWD, five mPAP, six cases of clinical worsening, five functional class amelioration, five serious adverse effects and abnormal LFT, and four deaths (Table 1).
Meta-analysis of the efficacy of bosentan for PAH
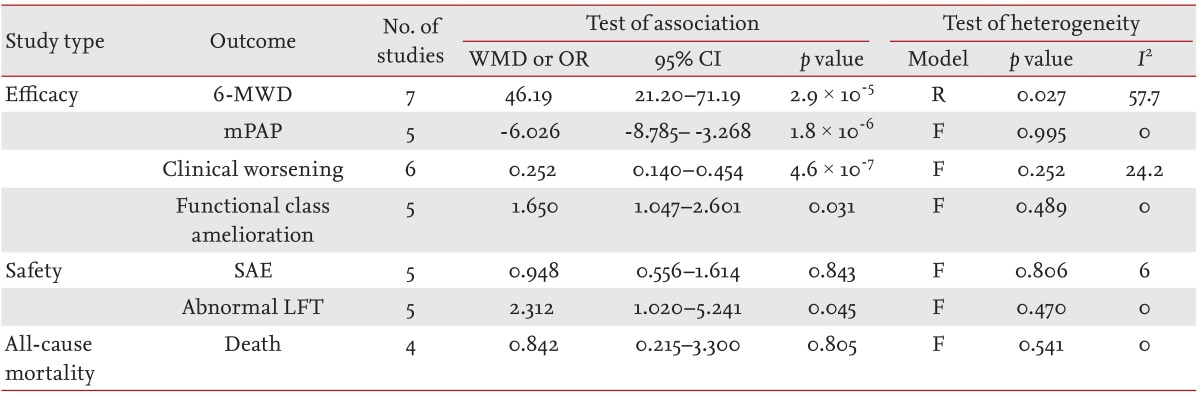
The 6-MWD was significantly higher in the bosentan group than in the placebo group (WMD, 46.19; 95% CI, 21.20 to 71.19; p = 2.9 × 10-5) (Table 2, Fig. 2). Compared with the placebo, bosentan significantly reduced the mPAP in patients with PAH (WMD, -6.026; 95% CI, -8.785 to -3.268; p = 1.8 × 10-6) (Table 2, Fig. 3). Clinical worsening was significantly lower in the bosentan therapy group than in the placebo group (OR, 0.252; 95% CI, 0.140 to 0.454; p = 4.6 × 10-7). Functional class amelioration was higher in the bosentan group than in the placebo group (OR, 1.650; 95% CI, 1.047 to 2.601; p = 0.031) (Table 2, Fig. 4). All of the efficacy outcomes were significantly improved in the bosentan therapy group compared with the placebo group (Table 2).
Meta-analysis of the safety of bosentan for PAH
The incidence of SAE was not different between the bosentan and placebo groups (OR, 0.948; 95% CI, 0.556 to 1.614; p = 0.843) (Table 2). However, LFT results were significantly more abnormal in the bosentan group than in the placebo group (OR, 2.312; 95% CI, 1.020 to 5.241; p = 0.045) (Table 2). All-cause mortality was not different between the bosentan and placebo groups (OR, 0.842; 95% CI, 0.215 to 3.300; p = 0.805) (Table 2).
Heterogeneity and publication bias
Between-study heterogeneity was not found during meta-analyses, except for analysis of 6-MWD (Table 2). Correlating the funnel plot was difficult, as the number of studies included in the analysis was small. However, no evidence of publication bias was identified (Egger regression test p > 0.1) (Fig. 5).
DISCUSSION
Two endothelin receptor subtypes mediate the effects of ET-1 [3,23]: the first, ETA, is preferentially expressed in vascular smooth muscle cells and fibroblasts and stimulates the vasoconstrictive and promitotic effects of ET-1 [3]. The receptor subtype, ETB, can be found either in vascular smooth muscle, where it induces vasoconstriction, or in the vascular endothelium, where it mediates vasodilation and clearance of circulating ET-1 [3,24]. Bosentan, a dual ET-1 receptor antagonist, is approved by the U.S. Food and Drug Administration for patients in WHO functional classes III and IV to improve exercise ability and reduce the rate of clinical worsening [23]. Patients taking bosentan are required to undergo monthly LFTs [23].
The present meta-analysis demonstrated that treatment with bosentan significantly improves the clinical outcome of PAH. The 6-MWD is a reliable tool to asses exercise capacity in patients with PAH. The effect of bosentan on exercise capacity as assessed by 6-MWD was significant. Bosentan therapy significantly reduces the incidence of clinical worsening and improves functional class amelioration and hemodynamic status markers, such as the mPAP. Significant improvements were detected for all of the efficacy endpoints. In the safety profile, the SAE was not increased in the bosentan group, but the incidence of abnormal LFT was higher in the bosentan group compared with the placebo group. Bosentan was not associated with significant changes in mortality compared with the placebo. The reasons behind the SAE and mortality findings are not clear; our findings may be due to the small samples of patients and studies, or the relatively short trial duration.
An important finding of the present meta-analysis was the significant improvements in all efficacy endpoints for bosentan. Statistically significant improvements in the 6-MWD, mPAP, and functional class amelioration were identified in this meta-analysis. Also, the frequency of clinical worsening was significantly lower in the Bosentan group than in the placebo group. The 6-MWD was used as a composite endpoint for exercise capacity. The WMD of the 6-MWD in the bosentan therapy group was 46.19 m compared with the placebo group, suggesting that bosentan treatment improved the symptoms of patients with PAH. The incidence of SAE was similar in the bosentan and placebo groups, suggesting that bosentan therapy is safe and well tolerated by patients with PAH even though mortality rates were not improved.
This meta-analysis differs from the meta-analysis of bosentan for PAH by He et al. [15]. In the present study, we focused on bosentan therapy and two additional studies of bosentan that included an extra 93 PAH patients and 96 controls, and the meta-analysis included more patients with abnormal LFT results. However, the results of this meta-analysis regarding the efficacy and safety of bosentan for PAH agree with those of the previous study.
The present study has some shortcomings that should be considered. First, most of the studies included in the meta-analysis had small sample sizes and short follow-up periods. In addition, the studies were not designed to evaluate the long-term effects of bosentan on mortality or its adverse effects. The observation times in the majority of trials were short (commonly 12 to 26 weeks, except one study that lasted 18 months). Therefore, this meta-analysis of bosentan may have been unable to detect significant differences in SAE and mortality, and the effect of bosentan on long-term safety and mortality is uncertain. Longer follow-up trials with larger sample sizes are needed. Second, the studies included in the meta-analysis were heterogeneous in terms of etiology. However, we could not perform additional subgroup analysis due to limited data.
In conclusion, this meta-analysis showed that bosentan therapy efficiently improved symptoms and hemodynamics in patients with PAH. In addition, bosentan therapy was safe and well tolerated. Although the long-term efficacy and safety of the medication must be more fully established, bosentan confers therapeutic benefits in patients with PAH. Further long-term studies are needed to adequately assess its efficacy and safety.
KEY MESSAGE
1. This meta-analysis shows that bosentan therapy is efficient for improving symptoms and hemodynamics in patients with pulmonary arterial hypertension.
2. Bosentan therapy was safe and well tolerated, although bosentan increased the incidence of abnormal liver function test compared with placebo.
3. Further long-term studies are needed to adequately assess the efficacy and safety of bosentan.
Acknowledgments
This study was supported by a Korea University Grant.
Notes
No potential conflict of interest relevant to this article is reported.