The start of chemotherapy until the end of radiotherapy in patients with limited-stage small cell lung cancer
Article information
Abstract
Background/Aims
Chemotherapy combined with radiation therapy is the standard treatment for limited stage small cell lung cancer (LS-SCLC). Although numerous studies indicate that the overall duration of chemoradiotherapy is the most relevant predictor of outcome, the optimal chemotherapy and radiation schedule for LS-SCLC remains controversial. Therefore we analyzed the time from the start of any treatment until the end of radiotherapy (SER) in patients with LS-SCLC.
Methods
We retrospectively analyzed 29 patients diagnosed histologically with LS-SCLC and divided them into two groups: a short SER group (< 60 days) and a long SER (> 60 days) group. Patients were treated with irinotecan-based chemotherapy and thoracic radiotherapy.
Results
Sixteen patients were in the short SER group and 13 patients were in the long SER group. Short SER significantly prolonged survival rate (p = 0.03) compared with that of long SER. However, no significant differences in side effects were observed.
Conclusions
Short SER should be considered to improve the outcome of concurrent chemoradiotherapy for LS-SCLC.
INTRODUCTION
Small cell lung cancer (SCLC) represents 13% of all newly diagnosed cases of lung cancer [1], and only one-third of patients with SCLC present with limited disease [2]. Although the incidence of SCLC has decreased in recent years, it remains a therapeutic challenge, as survival in patients with limited disease has not changed markedly over the past 10 years, reaching approximately 20% to 25% at 5 years in the best published series of patients treated with a multimodality approach [2-4]. Thoracic radiotherapy has established efficacy in limited stage (LS)-SCLC; however, the optimal combination of chemotherapy and chest radiation remains controversial [3,5]. Because SCLC cells proliferate rapidly, a potential hazard for tumor repopulation exists during a local control treatment course. If repopulation is rapid, the number of viable cells increases despite the cells being sensitive to chemotherapy [6]. This kind of potential hazard during a local control treatment course has been widely addressed, particularly for head and neck squamous cell carcinoma [7,8]. Accelerated proliferation of tumor clonogens was found to affect the outcome of head and neck squamous cell carcinoma during radiotherapy in a literature review [9] and in randomized controlled trials [10,11]. According to those reports, the overall response to treatment depends on the ratio between tumor cell death induced by each treatment and the rate of repopulation. Thus, we hypothesized that the overall duration of chemoradiotherapy is the most adequate predictor of the outcome of LS-SCLC. Therefore, we considered the time between the start of any treatment and the end of chest radiotherapy (defined as SER) as a quantitative measure that reflects proliferation of malignant cells in the primary cancer. We evaluated the prognostic values of the SER during management of LS-SCLC.
METHODS
Patients
The base population consisted of patients who were diagnosed with SCLC at the Korea University Medical Center from March 2001 to February 2008. Data were based on the most recent medical records of the patients, who were retrospectively reviewed.
Patients ≥ 18 years who had histologically or cytologically proven SCLC and stages qualified as LS were included. All patients had measurable lesions, adequate organ function but no prior radiotherapy, chemotherapy, or surgery. We defined limited disease as cancer confined to one hemithorax including contralateral, mediastinal, and hilar lymph nodes as well as ipsilateral and/or bilateral supraclavicular involvement but excluding malignant pleural effusion [12]. Patients had an Eastern Cooperative Oncology Group performance status of 0 to 2. Adequate organ function (bone marrow, liver, and kidney) was defined as a leukocyte count of at least 4,000/mm3, a platelet count of at least 100,000/mm3, a hemoglobin level of at least 9.5 g/dL, aspartate aminotransferase and alanine aminotransferase levels no greater than 100 IU/mL, serum creatinine level no greater than 1.2 mg/dL, and creatinine clearance of at least 60 mL/min.
Definition of SER
SER is defined as the time from the start of any treatment to the end of chest irradiation [13]. The patients were classified into two groups of short SER (< 60 days) and long SER (> 60 days).
Chemotherapy
Chemotherapy for the 29 patients on the standard protocol was based on irinotecan plus platinum. Cisplatin and carboplatin were comprised in platinum. Chemotherapy consisted of 4-week cycles of administering irinotecan (60 mg/m2/day intravenously) on days 1, 8, and 15 and cisplatin (60 mg/m2/day intravenously) or carboplatin based on the Calvert formula (area under the time-concentration curve [AUC] × (glomerular filtration rate + 25) mg/day) for a target AUC value of 5 mg/mL/min on day 1. All patients were prehydrated and received antiemetic drugs.
Radiation therapy
Patients received conventionally fractionated radiotherapy at individual doses of 180 cGy per day. A total dose of 5,580 cGy was delivered and was administered on weekdays. Radiation volumes and fields were individualized for each patient based on a recent chest computed tomography (CT) scan. Three-dimensional conformal radiotherapy techniques were used in all patients, and their targets were defined in accordance with the International Commission on Radiation Units and Measurements report, as follows. Gross tumor volume included pretreatment gross tumor volume and lymph nodes > 1 cm in the short axis diameter observed on CT scans. Clinical target volume included gross tumor volume and uninvolved mediastinal and ipsilateral hilar nodes. Planning target volume included clinical target volume plus a 10- to 15-mm margin. Elective irradiation of uninvolved supraclavicular fossa was not recommended. Three coplanar isocentric fields were used routinely to adequately cover the target volumes and to minimize doses to the lungs and other healthy tissues (e.g., spinal cord, esophagus, etc.). Prophylactic cranial irradiation was performed for patients who were in complete remission (CR) after completion of chemotherapy. Radiation therapy was completed before second-line chemotherapy was initiated.
Assessments and response evaluations
Before starting treatment, all patients underwent a complete blood count, renal and liver function tests, urinalysis, chest X-rays, chest CT scans, abdominal ultrasonography, brain magnetic resonance imaging, and a radionuclide bone scan.
Patient response was evaluated after every two cycles of chemotherapy by chest CT scan. The response was divided into the following four groups according to the with Response Evaluation Criteria in Solid Tumors (RECIST) criteria [14]: CR, partial response, stable disease, and progressive disease.
Toxicity scoring
Toxicity due to treatment was scored according to the National Cancer Institute Common Terminology Criteria for Adverse Effects version 3.0 [15]. The administration of irinotecan was omitted on days 8 or 15 if the leukocyte count was < 2,000/mm3 or if the platelet count was < 50,000/mm3. Administration of subsequent cycles of irinotecan was allowed when the leukocyte count increased to at least 3,500/mm3 and the platelet count increased to at least 100,000/mm3. The doses of irinotecan and cisplatin for subsequent cycles were reduced to 75% of the planned dose if grade 3 or higher hematological toxic effects or grade 2 or higher diarrhea was observed. The dose of cisplatin was reduced to 75% of the planned dose for patients suffering grade 2 or higher renal toxicity.
Statistics
Survival time was analyzed using the log-rank test as a Kaplan-Meier estimation method. Odds ratios (ORs) for survival were measured by Cox regression. Response was analyzed using the chi-square test. Survival time was measured from the date of initiating treatment to the date of death. The data analysis was carried out with SPSS software version 14.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Patients' characteristics
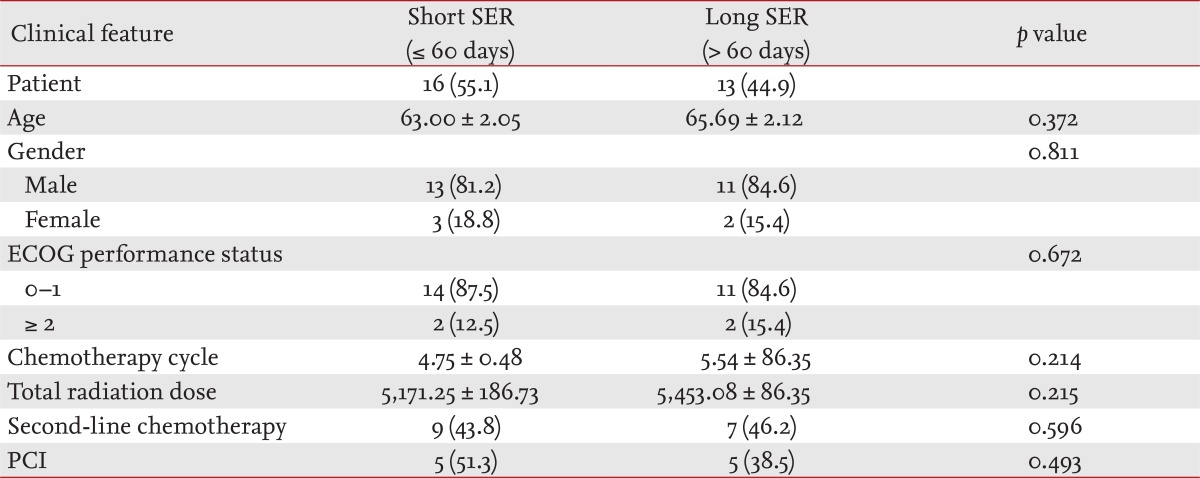
The characteristics of the 29 patients enrolled are listed in Table 1. Of the enrolled patients, 16 (55.1%) were in the short SER (≤ 60 days) group, and 13 (44.9%) were in the long SER (> 60 days) group. The clinical characteristics of the two groups were similar.
Treatment outcomes
The long SER patients had a shorter survival time than that of the short SER patients (median survival time, 513.83 ± 78.80 months vs. 810.06 ± 102.09 months; p = 0.0283) (Fig. 1). Many factors can influence survival time of patients, such as SER, patient performance status, chemotherapy dose, and total radiation dose. We evaluated the effect of such variables on survival time using the OR. The short SER group showed a 3.094-fold increased survival time compared to the long SER group. However, neither chemotherapy dose (p = 0.195) nor radiation dose (p = 1.000) affected patient survival time.
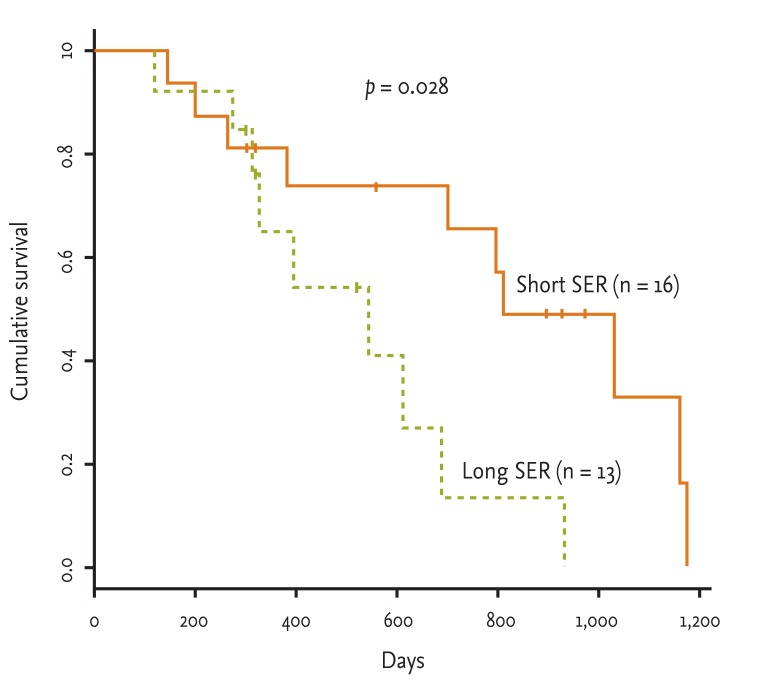
Overall survival time of patients with limited stage small cell lung cancer who were assigned to the short or long start of any treatment until the end of radiotherapy (SER) groups (median survival time, short SER vs. long SER; 810.1 ± 102.1 days vs. 513.8 ± 78.8 days).
Patients with LD-SCLC had a similar treatment response in both groups (p = 0.415) (Table 2). Neither low SER nor high SER patients achieved CR under the treatment regimen.
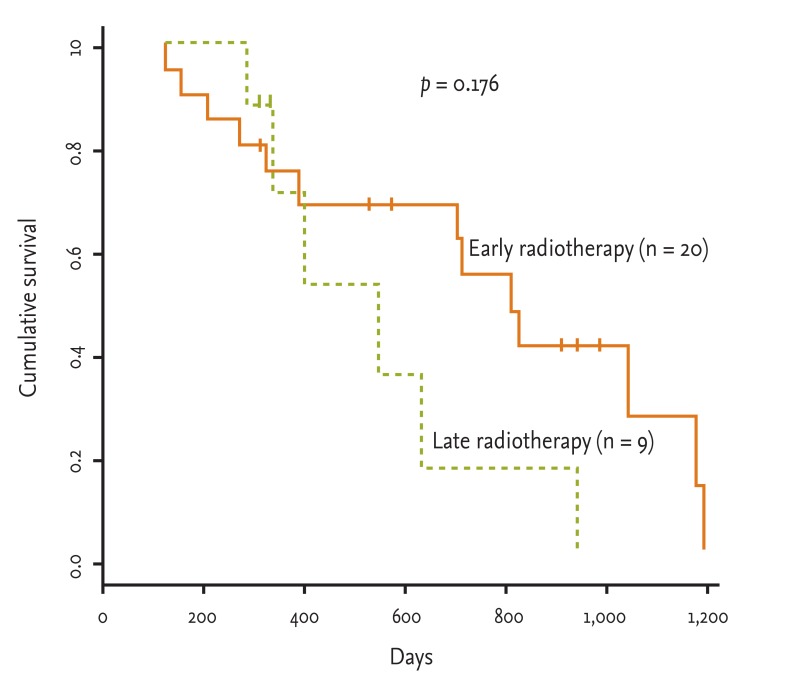
Some reports of concurrent early versus late radiotherapy have been published. Thus, we evaluated the radiation effect as the time between the start of chemotherapy and the start of radiotherapy (early, < 30 days; late, > 30 days). Twenty patients were eligible for early radiation therapy in the concurrent approach, compared to nine in the late group. However, no difference was observed between the two groups in terms of median survival time (early radiotherapy vs. late radiotherapy, 799 ± 100 days vs. 541 ± 119 days, respectively; p = 0.1764) (Fig. 2).
Toxicity
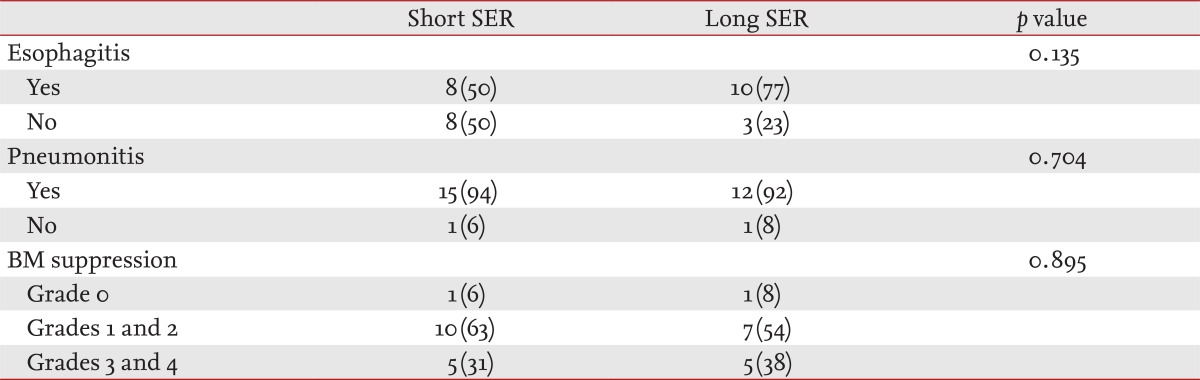
We evaluated local and hematological toxic effects. The common local effects due to radiation therapy were radiation esophagitis and radiation pneumonitis, but no differences were observed between the two groups (p = 0.135, esophagitis; p = 0.704, pneumonitis) (Table 3). We measured hematological toxicity as neutropenia and thrombocytopenia and categorized them as grades 0, 1, 2, 3, or 4. No differences were found for most of the hematological effects (p = 0.895).
DISCUSSION
De Ruysscher et al. [13] reported SER to be a novel parameter to rationally combine chemotherapy and radiotherapy trials. Such a report results from the hypothesis that accelerated proliferation of tumor clonogens is a well-recognized cause of treatment failure after radiotherapy and chemotherapy for several malignancies [7,9-11,16-20]. They considered that time factors may reflect both chemotherapy- and radiotherapy-associated accelerated proliferation of tumor clonogens. Before treatment, the lack of nutrients for large and dense tumors causes spontaneous cell loss, which counters cell growth. Once the treatment becomes effective, a large number of tumor cells are killed and removed. This results in reduced spontaneous cell loss due to malnutrition, which, in turn, results in accelerated treatment effects [21]. Therefore, the overall response to treatment will depend on the ratio of cells killed to the rate of repopulation. If repopulation is rapid, the number of viable cells will increase, despite being sensitive to chemotherapy [6]. Many studies have evaluated the efficacy of radiation therapy in terms of the timing, volume, dose, and fraction. Thoracic radiotherapy can be integrated with chemotherapy either concurrently or sequentially. In the concurrent approach, thoracic radiotherapy is delivered simultaneously with chemotherapy, either up front (early) or delayed (late) in the treatment cycle. The optimal integration of chemotherapy and chest radiotherapy in patients with LS-SCLC is unknown. However, the meaning of SER is quite different. SER is a quantitative measure of proliferation of cells in the primary tumor. Although early chest radiation is correlated with a short SER in many studies [22,23], the SER, a parameter that considers the time factor for both radiotherapy and chemotherapy, correlates more strongly with long-term survival than the timing of radiation [13]. The SER is a logical approach to investigation of the integration of radiotherapy and drugs because it takes into account not only whether a drug was administered concurrently or early during radiation, but also the time interactions when agents are delivered intermittently on days on which no radiotherapy was administered, or even when administered before the start of radiotherapy. We found no differences between early chemotherapy and late chemotherapy (p = 0.1764) (Fig. 2). However, when we compared short and long SER, a significant difference was observed between the two groups (p = 0.0283) (Fig. 1). When we assessed the effect of several factors, SER was found to be associated with overall survival time (OR, 3.094; 95% confidence interval, 1.08 to 8.84; p = 0.028).
SCLC is a relatively chemosensitive human solid cancer, and systemic chemotherapy is the mainstay of treatment; however, local recurrences occur in up to 90% of cases treated with chemotherapy alone [24]. Therefore, additional local irradiation can significantly improve local recurrence and overall survival rates [25]. Cisplatin and etoposide and/or cyclophophamide, doxorubicin (adriamycin), and vincristine regimens have been used worldwide as first-line chemotherapy for the past 20 years [26]. Furthermore, cisplatin and etoposide with concurrent thoracic radiotherapy have been used to treat patients with limited disease SCLC with good results [4,27]. A recent randomized phase III study comparing irinotecan plus cisplatin with cisplatin and etoposide for patients with extensive disease SCLC revealed a superior median survival rate and a superior 2-year survival rate for the irinotecan plus cisplatin combination therapy group [15]. Several recent studies have indicated that irinotecan plus platinum with concurrent radiotherapy is an effective and tolerable regimen for treating LS-SCLC [28,29]. Although further investigations of the irinotecan plus platinum regimen with concurrent radiotherapy for treating LS-SCLC are needed, its efficacy may be equivalent to that of the cisplatin and etoposide regimen. This is the first study to estimate the efficacy and tolerability of the irinotecan plus platinum regimen with thoracic radiotherapy based on the SER.
We found conclusively that a short time between the first day of chemotherapy and the last day of chest radiotherapy was associated with improved survival in patients with LS-SCLC. However, prospective trials and a meta-analysis should be conducted to confirm the value of the SER for patients with LS-SCLC.
KEY MESSAGE
1. Small cell lung cancer is a rapidly growing tumor. A potential hazard for tumor repopulation exists during a local control treatment. Therefore, the overall duration of chemoradiotherapy is the most adequate predictor of treatment outcome.
2. The short treatment duration between the first day of chemotherapy and the last day of chest radiotherapy is associated with improved survival in patients with limited stage small cell lung cancer.
Notes
No potential conflict of interest relevant to this article is reported.