Steroid Response in Refractory Asthmatics
Article information
Abstract
Inhaled glucocorticosteroids are currently the most effective anti-inflammatory controller medications for treating persistent asthma. The efficacies of glucocorticoids include reducing asthma symptoms, reducing exacerbation frequency, improving quality of life, improving lung function, decreasing airway hyperresponsiveness, controlling airway inflammation, and reducing mortality. However, the treatment response to glucocorticosteroids in asthmatics varies, and certain subtypes of asthma, such as refractory asthma, respond poorly to high-dose inhaled glucocorticoid and systemic steroids. The medical costs of treating refractory asthmatics represent about 50% of the total healthcare cost for asthma. A thorough understanding of the mechanisms of glucocorticoid action, patient responses to glucocorticoids, and steroid resistance observed in refractory asthmatics is necessary for the targeted development of therapeutic drugs. This review discusses the characteristics of severe refractory asthmatics and the mechanisms of steroid response and resistance in asthma treatment.
INTRODUCTION
Asthma is a chronic inflammatory airway disease involving episodic breathlessness and wheezing with airway hyperresponsiveness to environmental stimuli [1-3]. Airway inflammation in asthmatic patients is characterized by degranulated mast cells, infiltrating eosinophils, and an increased number of activated helper T-lymphocytes [1-3]. In asthma, many different inflammatory mediators such as lipid mediators, inflammatory peptides, chemokines, cytokines, and growth factors are increased [1-3]. The structural cells of the airways, including epithelial cells, airway smooth muscle cells, endothelial cells, and fibroblasts, also are a major source of inflammatory mediators in asthma [3]. Airway remodeling in both large and small airways has been documented in all degrees of asthma severity [4]. Structural changes in airway remodeling include loss of epithelial integrity, thickening of the basement membrane, subepithelial fibrosis, goblet cell and submucosal gland enlargement, increased smooth muscle mass, decreased cartilage integrity, and increased airway vascularity [4-7]. Inhaled glucocorticoids are currently the most effective anti-inflammatory controller medications for the treatment of persistent asthma [1-3] in Korea [1,2] and are recommended by the Global Initiative for Asthma (GINA) guidelines [3].
The prevalence of asthma is about 5-10% of the general population. Of these, approximately 5-10% are severe refractory asthmatics who respond poorly to asthmatic drugs, including high-dose inhaled steroids [5-12]. Severe refractory asthmatics have persistent symptoms, frequent symptom exacerbation, and severe airway obstruction even when taking high-dose inhaled steroids.
The subtypes of severe asthma [13-18] include fatal or near fatal asthma, steroid-dependent asthma, steroid-resistant asthma, difficult-to-control asthma, poorly controlled asthma, brittle asthma, and irreversible asthma. Patients who do not reach an acceptable level of control at step 4 of the GINA guidelines (reliever medication plus two or more controllers) are defined as having difficult-to-control asthma [3]. The American Thoracic Society [16] has defined refractory asthma (RA) as followings. RA was defined by one or both major criteria and one minor criteria as followings: 1) major criteria: treatment with continuous or near continuous (> 50% of year) oral corticosteroids (CS), requirement for treatment with high-dose inhaled CS; 2) minor criteria: a) requirement for daily treatment with a controller medication in addition to inhaled CS, e.g., long-acting β-agonist, theophylline, or leukotriene antagonist, b) asthma symptoms requiring short-acting β-agonist use on a daily or near daily basis, c) persistent airway obstruction (forced expiratory volume in one second [FEV1] < 80% predicted, diurnal peak expiratory flow [PEF] variability > 20%), d) one or more urgent care visits for asthma per year, e) three or more oral steroid "bursts" per year, f) prompt deterioration with ≤ 25% reduction in oral or inhaled corticosteroid dose, g) near fatal asthma event in the past.
This review discusses the characteristics of severe asthma, including RA, and the mechanisms of steroid response and steroid resistance in the treatment of asthma.
CHARACTERISTIC OF SEVERE ASTHMA
Subtypes of difficult-to-control asthma (Table 1)
Brittle asthma is diagnosed in patients with severe and unstable asthma who maintain a wide variability of PEF despite high doses of inhaled steroids. Type 1 brittle asthma is characterized by a wide, persistent, and chaotic PEF variability (> 40% diurnal variation for > 50% of the time over a period of at least 150 days) despite considerable medical therapy. Type 2 brittle asthma involves sudden acute attacks without an obvious trigger and lasting less than 3 hours in patients with apparently normal airway function or well-controlled asthma.
Nocturnal asthma has an early morning dip and double dip. Premenstrual asthma is unstable asthma that occurs 2-5 days before menses. Steroid-resistant asthma is diagnosed in those rare patients who take high-dose steroids (10- to 14-day course of prednisone ≥ 20 mg twice daily), but experience less than 15% improvement from baseline FEV1. Steroid-dependent asthma is defined as asthma that can be controlled only with high doses of oral steroids; it may be part of a continuum with steroid-resistant asthma at the other extreme.
Acute and severe asthma are diagnosed in patients with acute or subacute exacerbation with dyspnea, wheezing, chest tightness, and decreased lung function. Status asthmaticus leads to acute respiratory failure and can cause death (fatal asthma). Severe asthma is classified according to cell type, e.g., eosinophils and neutrophils [18-21]. Eosinophilic asthma observed on bronchoalveolar lavage and mucosal biopsy causes decreased lung function and occasional near-fatal events.
Clinical characteristics of severe asthma
Severe asthma, including RA, represents 10-20% of asthma cases and is associated with more drug medications and more hospital visits and admissions than mild to moderate asthma. Mortality occurs in 3-35% of severe asthma cases, and the medical cost to treat severe asthma is more than 50% of the total medical cost for treating asthmatic patients [22].
Risk factors for severe asthma are genetic and environmental, including many kinds of aeroallergens, beta-blockers, and anti-inflammatory drugs. Gastroesophageal reflux disease can affect asthma symptoms through esophagopharyngeal reflux and aspiration. In addition, factors such as denial, anxiety, fear, depression, socioeconomic status, and alcohol consumption can exacerbate asthma. Differential diagnoses [17] include smoking, chronic obstructive pulmonary disease, bronchiectasis, allergic bronchopulmonary aspergillosis, chronic infection, rhinosinusitis, vocal cord dysfunction, thyroid diseases, and inappropriate drug use. Among the genetic factors reported to be related to severe asthma are genetic variants of interleukin (IL)-4, IL-4 receptor, IL-9, IL-10, IL-1α, glutathione S-transferase, β2 receptor homozygous Gly-16 polymorphism, transforming growth factor (TGF)-β1, monocyte chemotactic protein, leukotriene C4 synthase, and 5-lipoxygenase [23-26].
Pathophysiological characteristics of severe asthma
Severe asthma can be classified based on four different pathological aspects.
1) Persistent airway inflammation despite maximal medication occurs in one-half to two-thirds of patients with severe asthma. In these patients, an increase in T-lymphocyte activating mediator is seen on mucosal biopsy; increases in the innate response factor tryptase, TGF-β2, cysteinyl leukotrienes, and 15-hydroxyeicosatetraenoic acid are found in the bronchoalveolar lavage fluid; and a thickening of the subepithelial basement membrane is observed [27]. 2) Severe asthma can be categorized based on the number of neutrophils or eosinophils [28]. An increase in the number of neutrophils can result in bronchiolitis obliterans, and a decrease in the number of eosinophils can be caused by steroid treatment [19-21]. 3) Severe asthma is associated with high-grade airway remodeling characterized by basement membrane thickening, epithelial denuding, airway smooth muscle thickening, and angiogenesis [4-6]. Submucosal fibroblasts secrete TGF-β, eotaxin, IL-4, and IL-13, causing collagen deposition and basement thickening. Activated macrophages secrete immunoglobulin, collagen I, collagen III, tenascin, and fibronectin, leading to reticular basement thickening and subepithelial fibrosis [29]. Reticular basement thickening is related to asthma severity [30,31]. 4) Severe asthma can also be classified according to changes in both large and small airways [32].
MECHANISMS OF STEROID
Steroid responses
Glucocorticoids, the mainstay of asthma management, bind to glucocorticoid receptors (GRs) in the cytosol, thereby activating the translocation of GRs to the nucleus. In the nucleus, GR binds to specific gene regulatory elements to induce the expression of genes involved in regulating airway inflammation (Table 2) [33].
In a study of steroid responses in patients with moderate to severe asthma, the relationship between responsiveness and the FEV1% predicted and blood and sputum eosinophil levels prior to glucocorticoid inhalation were investigated [28]. In this study, 86 adult asthmatic outpatients with an initial FEV1 < 80% received a glucocorticoid inhalant (fluticasone propionate 1,000 µg/day) for 4 weeks. The percentage change in FEV1 after treatment was determined as [(FEV14 weeks - FEV1baseline) / FEV1baseline)] × 100. The asthmatic patients showing a change of > 12% in FEV1 (n = 46, 53.4%) after treatment had a significantly higher proportion of blood eosinophils and a lower FEV1 volume (in liters) prior to treatment. The percentage change in FEV1 correlated with the number of sputum eosinophils prior to glucocorticoid inhalation (r = 0.242; p < 0.05) and correlated inversely with the FEV1% predicted prior to glucocorticoid inhalation (r = -0.462; p < 0.001). Thus, the FEV1% predicted and blood and sputum eosinophil levels prior to glucocorticoid inhalation were associated with the responsiveness to inhaled glucocorticoids in patients with moderate to severe asthma.
There was a wide range of steroid responsiveness in asthmatics with moderate to severe asthma in the above study [28] (FEV1, -21 to 126.8%; forced vital capacity, -20 to 47%; forced expiratory flow, -55.1 to 95%) (Fig. 1). This emphasizes the importance of investigating the mechanisms responsible for steroid unresponsiveness in asthma treatment. Korean and GINA guidelines [1-3] recommend a treatment strategy according to asthma control status, but the guidelines do not address the non-responder group. Potential new drugs are necessary to reverse steroid unresponsiveness in controlling asthma symptoms in refractory asthmatics.

Change in forced expiratory volume in one second (FEV1) following glucocorticoid inhalation therapy for 4 weeks.
The following factors must be considered when using high-dose inhaled steroids or systemic steroids to treat RA : 1) method of drug delivery, e.g., steroid inhaler; 2) presence of environmental factors that may aggravate asthma symptoms; 3) possibility of co-morbid diseases such as vocal cord dysfunction, gastroesophageal reflux disease, and chronic sinusitis; 4) psychological factors and patient compliance in taking asthmatic drugs; 5) presence of infections (Chlamydia Mycoplasma); 6) possible failure in activation or rapid clearance of prednisolone; and 7) simultaneous administration of other medications such as rifampin, phenytoin, carbarmazepin, phenobarbital, and anticonvulsants.
Steroid resistance
Those rare patients with steroid-resistant asthma exhibit less than 15% improvement in baseline FEV1 after a 10- to 14-day course of high-dose steroids (prednisone ≥ 20 mg twice daily). Proposed mechanisms leading to steroid resistance in asthma include intrinsic defects in neutrophils and mast cells, airway structural abnormalities, increases in inflammatory mediators related to steroid receptors, decreases in steroid receptor number and/or binding capacity, increases in GR-β, transcriptional factor repression, existence of steroid-resistant neutrophils, imbalance between acetylation and deacetylation, and airway remodeling [6,34,35].
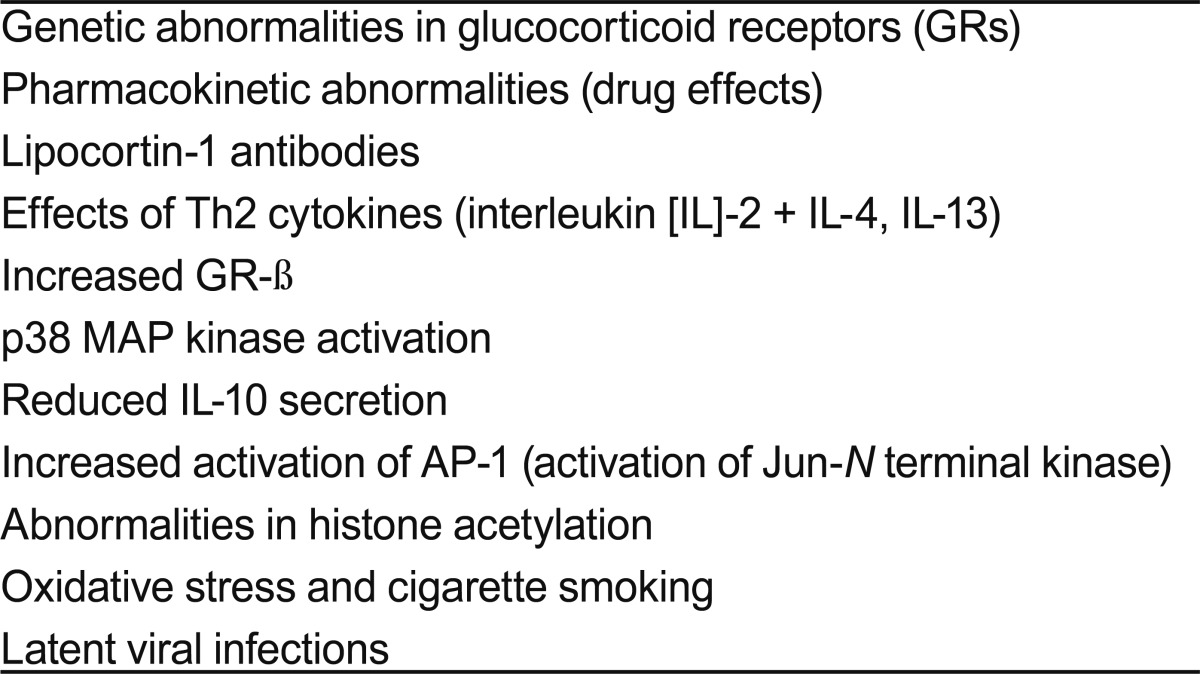
Factors that may contribute to steroid resistance are listed in Table 3 [35,36]. These include a decreased number and/or genetic variation of GR; abnormal GR binding capacity; decreased DNA-binding activity of GR; alterations in transcription factors such as AP-1; immune dysregulation related to cytokines, chemokines, IL-4, p50 nuclear factor-κB, or signal transducer and activator of transcription-4; mitogen-activated protein kinase phosphatase-2 single nucleotide polymorphism; increased neutrophils; viral infections; allergens; mycobacterial infections; and smoking.
NEW THERAPEUTIC DRUG TRIALS IN RA
Currently, clinical trials are evaluating several drugs for the treatment of severe asthma and RA, including methotrexate, gold, cyclosporine, intravenous gamma globulin, and macrolide antibiotics. However, the effects of these drugs on severe RA are minimal. In addition, phosphatidylinositol 3-kinase inhibitors, activated p38 mitogen-activated protein kinase inhibitors, and vitamin D3 to induce IL-10 production are undergoing therapeutic trials. Recent medical advances in the pathophysiological mechanism of asthma have led to the development of many asthma drugs [36-40]. New steroids, new bronchodilators, phosphodiesterase-4 inhibitors, transcription factor inhibitors, adhesion inhibitors, mediator antagonists, antioxidants, anti-IgE antibodies, cytokine inhibitors and antagonists, chemokine receptor inhibitors and agonists, and sublingual immunotherapy have been developed [41].
CONCLUSIONS
Glucocorticoids are mainstay therapeutic drugs for decreasing airway inflammation in asthma. Refractory asthmatics represent 5-10% of all asthmatics, but account for more than 50% of the total treatment cost of asthma. Understanding the pathophysiology of severe asthma, RA, and steroid-resistant asthma is necessary for the development of effective therapeutics. In order to develop individualized treatment approaches to severe RA, continued research is needed to identify genetic and environmental factors and to define the mechanisms of ongoing immune regulation and steroid responses.
Notes
No potential conflict of interest relevant to this article was reported.