Risk Factors of Cryptogenic Hepatocellular Carcinoma in Patients with Low Body Mass Index or without Metabolic Syndrome
Article information
Abstract
Background/Aims
Many patients are diagnosed with cryptogenic hepatocellular carcinoma (HCC) without metabolic syndrome (MS). We investigated the risk factors for cryptogenic HCC in patients with a low body mass index (BMI) or without MS.
Methods
Thirty-six patients were diagnosed with cryptogenic HCC over a 10-year period at a tertiary research hospital. Data including BMI score and risk factors for MS were analyzed retrospectively. Patients with fewer than two risk factors for MS (n = 16) were compared with those with two or more risk factors (n = 20). Patients with high BMI (≥ 23 kg/m2, n = 20) were also compared with those with lower BMI (n = 16).
Results
Patients with fewer than two risk factors for MS were significantly more likely to smoke and be hepatitis B surface antibodies (anti-HBs)-positive vs. patients with two or more risk factors. However, only smoking was statistically significant on multivariate analysis. Peaks of BMI were observed in two regions. Lower BMI was significantly associated with the presence of anti-HBs compared with high BMI, although this association was not statistically significant on multivariate analysis.
Conclusions
Smoking is a potential risk factor for cryptogenic HCC in patients without MS. Remote hepatitis B virus infection may be a risk factor for cryptogenic HCC in patients without MS or with a low BMI.
INTRODUCTION
The incidence of hepatocellular carcinoma (HCC) is high in South Korea, which is an endemic area of hepatitis B virus (HBV) infection. Most forms of HCC are related to hepatitis B, hepatitis C, or alcohol intake [1,2]. Since the discovery of the hepatitis C virus (HCV), the incidence of cryptogenic HCC has decreased. However, in about 5-30% of HCC cases, the cause is unknown. Many retrospective studies investigating HCC in the setting of cryptogenic cirrhosis have supported the notion that nonalcoholic fatty liver disease (NAFLD) is one of the causes of cryptogenic cirrhosis and can progress to HCC [3,4]. NAFLD reflects a constellation of metabolic syndrome components, including glucose intolerance, hypertension, dyslipidemia, and obesity. Cryptogenic HCC is diagnosed when an extensive evaluation has excluded recognizable etiologies of chronic liver disease [5-7]. In South Korea, the incidence of cryptogenic HCC is increasing as cases of obesity and type II diabetes mellitus increase [1,8,9]. In Japan where obesity is rare, studies have reported that the features of cryptogenic HCC are related to components of metabolic syndrome such as obesity and glucose intolerance [10]. Thus, the association between cryptogenic HCC and metabolic syndromes is well known.
However, in clinical practice, cryptogenic HCC may also occur in patients who are not obese and do not have metabolic syndrome. This study was conducted to investigate the risk factors for development of cryptogenic HCC in patients without metabolic syndrome or obesity.
METHODS
Patients and methods
Thirty-six patients diagnosed with cryptogenic HCC at a tertiary referral university hospital (Ilsan Paik Hospital, Inje University College of Medicine, Goyang, Korea) from January 2000 to December 2009 were included in the study. The diagnostic criteria for cryptogenic HCC were defined as HCC that is not related to hepatitis B, hepatitis C, or alcohol intake history. Autoimmune hepatitis, genetic liver disease, and Budd-Chiari syndrome were also excluded. The diagnosis of HCC was based on the criteria of the Korean Liver Cancer Study Group and the National Cancer Center [11]. The presence of hepatitis B surface antigen (HBsAg) was set as the criterion for HBV hepatitis, and the presence of HCV antibody was the criterion for HCV hepatitis. Those with alcohol-associated HCC, defined as male patients whose daily alcohol intake was 40 g or more for 10 years and female patients whose daily alcohol intake was 20 g or more for 10 years or more [12], were excluded from the study. The medical records of enrolled patients were retrospectively analyzed.
A patient with glucose intolerance was defined as one whose glucose level was 110 mg/dL in at least two tests or a patient who was receiving diabetic medication or insulin treatment [13]. A patient with hypertension was defined as one who was taking an anti-hypertensive drug or whose blood pressure was > 140/90 mmHg [14]. A patient with dyslipidemia was defined as one whose triglyceride (TG) level was > 150 mg/dL, who was receiving treatment for TG, or whose high-density lipoprotein level was < 40 mg/dL (male) or < 50 mg/dL (female) [14]. A smoker was defined as one who had a history of smoking of more than 20 pack-years [15]. In all patients, a biochemical test was performed and the Child-Pugh (CP) scores were calculated.
The body mass index (BMI) of the patients was calculated, and patients were divided into those with BMI ≥ 23 kg/m2 or more and those with BMI < 23 kg/m2, according to the criterion of 23 kg/m2 BMI for overweight Asian males [16]. The incidence of glucose intolerance, hypertension, dyslipidemia, smoking history, other cancer history, and hepatitis B surface antibodies (anti-HBs) positivity were compared.
The patients were also divided into two groups according to the number of risk factors they had for metabolic syndrome. These consisted of glucose intolerance, hypertension, dyslipidemia, and obesity, defined as BMI greater than 25 kg/m2 [14]. The two groups were compared based on their smoking or non-smoking habit, anti-HBs status, other cancer history, and clinical data to identify the risk factors for HCC in these patients.
The study protocol was approved by the Institutional Review Board of Ilsan Paik Hospital, Inje University College of Medicine, Goyang, Korea.
Statistical analysis
SPSS version 11.0 (SPSS Inc., Chicago, IL, USA) was used to perform all analyses. The Pearson χ2 test was used to compare the qualitative variables of those with a BMI of 23 kg/m2 or more and those with a BMI of less than 23 kg/m2, and of those who had two or more of the four factors constituting metabolic syndrome (glucose intolerance, hypertension, dyslipidemia, and a BMI ≥ 25 kg/m2) with those who had fewer than two. Student's t test was used to compare quantitative variables.
Variables that were significant by univariate analysis were tested by multivariate logistic regression to determine independent risk factors for cryptogenic HCC. Differences were considered significant at a two-sided p ≤ 0.05.
RESULTS
Baseline characteristics of the 36 cryptogenic HCC patients
The 36 patients included 26 males and 10 females with a mean age of 72.4 ± 11.0 years (range, 48 to 88). The mean BMI was 24.1 ± 4.2 kg/m2 (range, 15.8 to 32.4), and 20 (55.6%) patients had a BMI ≥ 23 kg/m2 (above the criterion for overweight). Detailed baseline clinical characteristics are shown in Table 1.
Fourteen (38.9%) patients had glucose intolerance, 21 (58.3%) hypertension, 14 (38.9%) dyslipidemia, and 11 were smokers. Eleven patients were anti-HBs-positive.
Comparison of clinical characteristics for cryptogenic HCC according to the number of metabolic syndrome risk factors
The patients were divided into those with two or more of the four factors constituting metabolic syndrome (glucose intolerance, hypertension, dyslipidemia, and BMI ≥ 25 [obesity]) and those with fewer than two. Although there was no significant difference in smoking habit, other cancer history, or level of alpha-fetoprotein between the two groups, the rate of anti-HBs positivity was significantly higher in the group without metabolic syndrome (58.3 vs. 21%, respectively; p = 0.026). Additionally, smoking was significantly higher in the group without metabolic syndrome (50% vs. 15%, respectively; p = 0.034) (Table 2). In a logistical regression analysis including anti-HBs and smoking as variables, anti-HBs positivity rate did not significantly differ between the two groups (odds ratio [OR], 0.29; 95% confidence interval [CI], 0.21 to 4.15; p = 0.368), whereas smoking did differ significantly between the two groups (OR, 10.25; 95% CI, 1.49 to 70.43; p = 0.018) (Table 3).
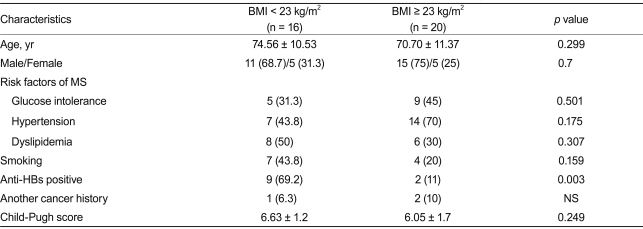
Comparison of clinical characteristics between BMI ≥ 23 kg/m2 and BMI < 23 kg/m2 groups
The peak incidence of BMI was observed in two regions (18.5-22.9 kg/m2 and 25-29.9 kg/m2) (Fig. 1). Twenty (55.6%) patients had a BMI of 23 kg/m2 or more, and 16 (44.4%) patients had a BMI < 23 kg/m2. There was no significant difference between the two groups in terms of glucose intolerance, hypertension, dyslipidemia, presence of another cancer, smoking habit, or CP score (Table 4). The number of anti-HBs-positive patients was significantly higher in the BMI < 23 kg/m2 group on univariate analysis (69.2% vs. 11.1%, respectively, p = 0.003) (Table 4). A binary logistic regression analysis that included age, hypertension, smoking habit, and anti-HBs found no statistically significant difference between groups in these variables, but smoking had an odds ratio of 6.42 for cryptogenic HCC in the low-BMI group, which suggests the possibility of a relationship (Table 5).

The distribution of body mass index in cryptogenic hepatocellular carcinoma patients. Peak body mass index incidence was observed in two regions (18.5-22.9 kg/m2 and 25-29.9 kg/m2).
DISCUSSION
HCC is the most rapidly increasing cause of cancer death globally. It is estimated that half a million cases occur annually worldwide, making HCC the fifth most common malignancy in men and the ninth in women. Most HCC cases are fatal; thus, its incidence rate is very close to its mortality rate [17].
The well-known causes of HCC include HBV, HCV, and alcohol. The socioeconomic damage due to HCC is considerable in countries such as South Korea, where hepatitis B is endemic [18]. NAFLD, one of the pathophysiologies of metabolic syndromes, is another cause of HCC [5]. Thus, extensive studies on non-alcoholic steatohepatitis (NASH) and NAFLD for cryptogenic HCC are in progress [19,20].
NAFLD is one of the most common causes of abnormal liver enzyme levels in adults. It is marked by a generalized triglyceride deposition within the hepatic parenchyma that pathologically resembles the macrovesicular steatosis seen in alcoholic liver disease, but occurs in subjects without considerable alcohol consumption [21]. Several studies have examined the clinical progression from NAFLD to cirrhosis, HCC, and liver-related death [22,23]. As NAFLD may account for a substantial proportion of cases of cryptogenic cirrhosis, the occurrence of HCC among these patients was also examined. The prevalence of obesity and diabetes is significantly higher in patients with cryptogenic HCC compared with those with HCC associated with viral- or alcohol-related cirrhosis [24].
HCC also occurs, however, in patients whose condition is not related to NAFLD. It is meaningful that this study found a high rate of smoking and anti-HBs positivity in the patient group with BMIs < 23 kg/m2, a level that is defined as a low BMI, and in those without metabolic syndrome. Smoking is also a cause of various cancers. Various carcinogens from smoking can cause cancer through direct contact with human organs or through immunological mechanisms or indirect metabolites that do not have direct contact with the organs [25]. Although the effect of smoking on the liver remains controversial, recent studies showed that smoking is a risk factor for HCC and a synergic risk factor with alcohol and obesity [15,19]. Additionally, one study reported that smoking could decrease the treatment response to interferon in chronic hepatitis C [26]. Our data indicate that smoking may be an independent risk factor for HCC in patients with cryptogenic HCC who do not have metabolic syndrome (Tables 2 and 3).
Studies have been conducted to identify the causes of cryptogenic HCC in patients who do not have hepatitis B or C and who are non-alcoholic. In particular, in East Asia, where the prevalence of hepatitis B is high, reports on the relationship between hepatitis B and cryptogenic HCC are constantly emerging. By investigating the status of viral markers in HBsAg-negative patients with HCC, studies have found that a history of or infection with occult hepatitis B could cause HCC [20,27,28]. The integration of occult HBV into the human genome caused mutation of the p53 gene and induced hepatocarcinogenesis [29].
This study has several limitations. First, we did not determine whether the patients with HCC had a resolved past HBV infection or had been vaccinated. Additionally, as HBV DNA PCR was not performed, the HBV infection status could not be determined.
This study is meaningful because we found that smoking is a risk factor for patients with HCC whose BMI is low and who do not have metabolic syndromes. Furthermore, this study suggests a relationship with past HBV infection. Further studies are required to ascertain whether past and/or occult HBV infection can integrate into the human genome in low-BMI patients to a greater degree than in patients with an above normal body weight.
Acknowledgments
This work was supported by a grant from Inje University, 2009.
Notes
No potential conflict of interest relevant to this article was reported.