Endoscopic Therapy in Chronic Pancreatitis
Article information
Abstract
Chronic pancreatitis (CP) is a debilitating disease that can result in chronic abdominal pain, malnutrition, and other related complications. The main aims of treatment are to control symptoms, prevent disease progression, and correct any complications. A multidisciplinary approach involving medical, endoscopic, and surgical therapy is important. Endoscopic therapy plays a specific role in carefully selected patients as primary interventional therapy when medical measures fail or in patients who are not suitable for surgery. Endoscopic therapy is also used as a bridge to surgery or as a means to assess the potential response to pancreatic surgery. This review addresses the role of endoscopic therapy in relief of obstruction of the pancreatic duct (PD) and bile du ct, closure of PD leaks, and drainage of pseudocysts in CP. The role of endoscopic ultrasound-guided celiac plexus block for pain in chronic pancreatitis is also discussed.
INTRODUCTION
Chronic pancreatitis (CP) is a continuous inflammatory disease of the pancreas characterized by irreversible morphological changes that typically cause pain and/or permanent loss of function [1]. Patients with chronic pancreatitis are also at risk for developing pancreatic cancer. The aim of endoscopic therapy is to control symptoms, prevent progression of the disease, and correct complications.
Pain is one of the main symptoms of chronic pancreatitis, and its etiology is multifactorial. Pain can result from increased pressure in the main pancreatic duct (PD) leading to intraparenchymal/interstitial hypertension or from peripancreatic/celiac neural inflammation [2,3]. Intraductal hypertension occurs primarily due to obstruction of pancreatic juice outflow from PD strictures, intraductal stones, decreased compliance of the main PD, and major/minor papillary sphincter stenosis [4]. Other factors that may indirectly contribute to pain include the complications of CP, such as pseudocysts, PD leaks/ascites, and biliary and duodenal obstruction.
The role of endoscopic therapy in CP to alleviate pain by reducing outflow obstruction of the PD and by decreasing ductal hypertension has recently become the subject of debate. Two studies comparing endoscopic therapy to surgery have shown that surgical outcomes for pain relief were more durable [5,6]. However, the subjectivity of pain assessment, lack of blinding, and small sample size in these studies may have led to bias. Additionally, the specific disease characteristics, such as the presence of multiple pancreatic stones and the locations of the strictures, were not clearly described in the surgical group, which suggests there was a possibility of chance randomization of more refractory patients to the endoscopy treatment group. Hence, some authors [7] have suggested that endoscopic management plays a specific role in carefully selected patients as primary interventional therapy when medical measures fail or in high-risk surgical candidates. Endotherapy for CP is also utilized as a bridge to surgery or as a means to assess potential response to pancreatic surgery [8]. Finally, endotherapy is employed selectively by the authors for treatment of recurrent abdominal pain or complications after pancreatic surgery for CP. Other endoscopic therapies to relieve pain include the use of endoscopic ultrasound (EUS) to provide EUS-guided celiac block for CP.
The role of endotherapy in improving pancreatic function is less clear, with a large multicenter study [9] showing no improvement in pancreatic function. However, one secretin-enhanced magnetic resonance cholangiopancreatography study suggested that pancreatic exocrine function can improve after endoscopic therapy [10], and another study showed that the development of clinical steatorrhea was delayed for about 10 years when compared with the natural history of chronic pancreatitis [11].
PANCREATIC ENDOTHERAPY
The principle of pancreatic endotherapy is to alleviate the outflow obstruction to exocrine juice flow. This is based on the assumption that PD hypertension is the cause of the symptoms. Failure to respond to endoscopic therapy suggests that PD hypertension is not the primary cause of the patient's symptoms or that the intraductal pressure was not adequately reduced to achieve clinical benefit.
Main PD deep cannulation is the first crucial step to successful pancreatic endotherapy. With the endoscope in the short position and the papilla viewed en face, the main PD can be accessed with a regular cannula at the 1-4 o'clock position of the native major papilla orifice and at the 5 o'clock position of the papilla with prior biliary sphincterotomy. If minor papilla cannulation is indicated, it is typically achieved with the endoscope in the long position using a highly tapered catheter with a 0.46 mm and 0.53 mm guide wire. Secretin administration to promote pancreatic juice flow and/or methylene blue flushes over the papilla may help in orifice identification and cannulation [12,13]. In cases where cannulation is not achieved despite the use of the above described methods, EUS-guided pancreatography has been described for both identification of the desired papillary orifice and successful PD drainage [14-16]. In failed main PD deep cannulation due to obstruction or extreme tortuosity of the ventral duct, access to the main PD may be obtained via the minor papilla when the minor papilla and accessory duct are patent. Once deep cannulation is acquired with placement of wires, specific endotherapy can be performed as detailed below.
PANCREATIC DUCT STRUCTURES
PD strictures are typically benign and occur secondary to previous stone disease, inflammation, or fibrosis [17]. However, a retrospective series of 355 patients reported a 12% risk of malignancy in isolated PD strictures [18]. It is therefore crucial to have a high index of suspicion for malignancy in patients with PD strictures and obtain pancreatic imaging with dual-phase computed tomography (CT) scan and/or EUS prior to endoscopic retrograde cholangiopancreatography (ERCP). Tissue sampling of the stricture during ERCP should also be performed on the PD stricture in patients without risk factors for CP, symptoms such as weight loss and anorexia, or evidence of CP on pancreatography. Factors affecting success of endotherapy include location, the number and length of the strictures, and the presence of upstream dilation. PD strictures in the tail of the pancreas and multiple strictures along the length of the main PD are more difficult to manage by endotherapy. The best outcomes of endotherapy are achieved when strictures of the main PD in the head with upstream dilation are stented.
Technique of endotherapy
Pancreatic sphincterotomy (major and/or minor papilla) is frequently performed after cannulation and wire placement across the stricture. Stricture dilation is performed with a graduated dilating catheter or balloon dilators. One or more PD stents are advanced over the guide wire using a pusher tube. The choice of stent size is dependent on the size of the PD downstream of the stricture. Smaller ducts require 3-7 French stents, whereas more dilated PDs require 8.5-11.5 French stents. We prefer placing stents with an external pigtail and an internal flange to prevent proximal and distal migration, respectively. Occasionally, PD strictures are too tight or too angulated to allow passage of conventional dilators or catheters, and Familiari et al. [19] described the placement of a guide wire (used as a dilator) across these strictures for 24 hours. The mechanism of action is thought to be the presence of the guide wire across the stricture in combination with slight movements caused by breathing resulting in dilation of the stricture. Subsequent dilation and placement of a stent was successful. The timing of pancreatic stent exchange is variable. Farnbacher et al. [20] attempted to predict factors that resulted in clogging of PD stents. However, clinical and laboratory data did not reliably indicate clogging, and therefore stent exchange or removal was recommended within 3 months in high-risk patients. Delhaye et al. [21] showed that on-demand exchange was as effective as early replacement based on the theory that pancreatic juices can drain around the stent even when it is occluded. Currently, routine (6-12 weeks) and on-demand exchange based on recurrence of symptoms are used in clinical practice.
Results of endotherapy
Wilcox [22] summarized the results of PD stent placement for pain in chronic pancreatitis among 1500 patients treated in 15 series. Benefit was seen in 31-100% during a follow-up interval of 8-72 months. The greatest benefit was seen in patients with dominant strictures and dilated ducts [23,24]. In a recent review of nine series including a total of 491 patients undergoing stenting (5-11.5 Fr stents) for strictures, Nguyen-Tang and Dumonceau [25] reported early pain relief in 65-95% and sustained pain relief in 52-90% of cases. Patients were followed up over 14-69 months, and 4-26% of patients subsequently underwent operations after endotherapy. As with surgical decompressive procedures, it appears that the response to stenting is attenuated over time.
In a large multicenter study, 1,018 patients with CP [9] were followed prospectively for a mean of 4.9 years after endoscopic intervention. The study population included 478 patients with strictures alone. The remaining patients had stones alone or strictures with stones. All patients with strictures alone had pain initially, and 37% still had pain at follow-up. A significant reduction in pain (no or weak pain) was achieved in 84% of cases. Rates of pain relief were similar in patients with dominant strictures in the head and/or body, pancreatic stones in the head and/or body, a combination of stones and strictures, and complex pathology. It appears that complete stricture resolution is not mandatory for symptom improvement, which implies that luminal patency was sufficient or that other therapies performed along with stenting contributed to the benefit. In all of these studies, single plastic stents were usually sequentially changed at multiple ERCP sessions.
In terms of pain relief, the long-term efficacy of pancreatic stenting is uncertain, and multiple ERCPs are required for stent exchanges either at regular intervals or "on-demand" (i.e., when pain develops). Even with an on-demand schedule, the number of ERCP sessions approximates four or five in large studies [26,27], making this strategy relatively unsatisfactory. Costamagna et al. [28] attempted to address this issue by placing multiple pancreatic stents. Nineteen patients with dominant PD strictures in the pancreatic head who had pain relief by a single previously placed PD stent underwent balloon dilation of the stricture and placement of 8.5-10 French pancreatic stents 4-7 cm in length (median of three stents; range, 2 to 4 based on the tightness of the stricture) for 6-12 months. Stricture resolution was seen in 95% of patients at stent removal. After a median follow-up period of 38 months after stent removal, 84% remained pain-free, and PD stricture recurred in 10.5% of cases (two patients) and was subsequently treated with single stent placement. The main advantage of this technique was the low number of ERCP sessions (two) and large dilation diameter due to the multiple stents placed, which may account for the higher success rate. However, prospective controlled studies are required to confirm these findings. To date, there have been no trials comparing single vs. multiple stenting for PD strictures.
Attempts to reduce the number of procedures and improve the outcome of stenting with self-expandable metal stents (SEMS) have met with varying success. An early study by Eisendrath and Deviere [29] evaluated a partially covered SEMS for PD stricture. Unfortunately, after 6 months of treatment in 29 patients with 18 Fr metal stents 23 mm in length, most patients developed stent occlusion due to mucosal hyperplasia. Fully covered SEMS have recently become available and were thought to overcome the problem of mucosal hyperplasia. Sauer et al. [30] used a Viabil stent 8 mm in diameter (Conmed, Utica, NY, USA) in six patients who had persistent pain after plastic stenting of an MPD stricture. One patient had pancreatic cancer and was excluded from the study. Three of the remaining five patients who underwent stent removal at 3 months presented with early symptomatic stricture recurrence. Park et al. [31] used another model of fully covered SEMS (Niti D-type, Taewoong, Seoul, Korea) in 13 patients with recurrent pain and refractory MPD stricture after removal of pancreatic plastic stents. The stents were removed 2 months after insertion. SEMS migration was observed in five (38%) of 13 patients (including one case of proximal migration). Complications developed in five (30%) patients, including cholestasis and pancreatitis flares. During a median follow-up of 5 months after SEMS removal, no pain relapse was reported. The same group of authors recently published a larger study of 32 patients [32] using a modified version of the original stent, and all patients achieved pain relief from stent placement. There was no occurrence of stent-induced pancreatitis or pancreatic sepsis. None of the stents migrated, and all stents were easily removed. Follow-up ERCP 3 months after stent placement showed resolution of duct strictures in all patients. Pancreatograms obtained at fully covered SEMS removal displayed de novo focal pancreatic duct strictures in five patients, but all were asymptomatic. Although these studies are encouraging, further refinement in SEMS design and additional long-term data are needed.
Some natural history studies of CP [33,34] have shown spontaneous pain relief in late stages due to pancreatic "burn out." However, Lankisch et al. [35] showed that even after 10 years of follow-up of severe pancreatitis, more than 50% of patients continued to have pain. This variation in natural history can bias the results of outcome studies of endotherapy for pancreatic strictures due to lack of sham control procedure. Wilcox and Lopes [36] recently proposed a much-awaited trial studying pain and psychosocial outcomes in patients with symptomatic PD strictures who were randomized to pancreatic endotherapy or sham procedure. If less than 50% improvement in pain in patients randomized to sham procedure is observed, there will be crossover to the endoscopic intervention group, and otherwise they will be followed clinically for the proposed duration of 3 years. The results of this study are eagerly awaited.
PANCREATIC DUCT STONES
The prevalence of stones in CP ranges from 20 to 60% [9,37]. The majority of pancreatic stones are radioopaque due to calcium content, with a small percentage being radiolucent. Obstruction of the PD from stones with resultant ductal hypertension is thought to be one of the potential mechanisms responsible for attacks of acute pancreatitis or exacerbations of chronic abdominal pain in patients with CP. This suggestion is supported by studies showing that removal of stones using various methods results in symptomatic improvement.
Technique of endotherapy
Small pancreatic stones can be removed after pancreatic (major/minor) sphincterotomy (Fig. 1) using balloon or basket extraction. Using these standard techniques, the reported rate of success is approximately 50-75% [38]. However, if there is a stricture downstream (toward the duodenum) of the stone, it is usually necessary to dilate the stricture prior to attempting stone extraction. Sherman et al. [39] found that the following factors favored complete stone removal by endoscopic techniques alone: stones < 1 cm in size; presence of three or fewer stones; stones confined to the head and body; and absence of impacted stones or stones upstream (toward the tail) of a stricture. Stones that are larger, impacted, or upstream to a stricture frequently require fragmentation via mechanical lithotripsy, intraductal lithotripsy with a pulsed dye laser or electrohydraulic lithotripsy (EHL), or extracorporeal shockwave lithotripsy (ESWL) prior to attempted extraction. Among the three options for fragmentation, ESWL is the most utilized method, although there have been no comparative trials among different forms of lithotripsy.

Pancreatic duct stone removal. (A) Pancreatic duct stone at minor papilla. (B) Needle knife sphincterotomy over stone. (C) Passage of stone post-sphincterotomy. (D) Patent dorsal pancreatic duct post-sphincterotomy and stone removal with air bubbles seen in the duct.
The success rate of stone fragmentation with ESWL is usually > 90% [40], and it can be performed under moderate sedation or general anesthesia. Some authors recommend the use of general anesthesia, as pancreatic ESWL can be very painful. In some cases, general anesthesia can be used to assist ESWL in small stones that are difficult to target by limiting respiratory movements with decreased tidal volume [25]. Localization of the stones is done under ultrasound or fluoroscopy. However, previous studies have shown that ultrasound localization of pancreatic stones results in lower fragmentation rates [37,40]. Following ESWL, endoscopic removal of the stone fragments is performed in the same manner as for small stones, during the same or in a different session (based on the availability of a local lithotripter and personnel). ESWL alone without the need for ERCP has been shown in large uncontrolled series [41,42] to relieve pain in calcifying CP. This was confirmed in a large prospective randomized controlled trial, which showed spontaneous passage of stone fragments after ESWL without the need for endoscopic removal [43].
Large radiolucent pancreatic stones that cannot be targeted by fluoroscopy for ESWL can be removed via mechanical lithotripsy or intraductal lithotripsy with pulsed dye laser or EHL. Mechanical lithotripsy requires stone capture in a basket, which may be difficult for impacted stones. Mechanical lithotripsy has been reported to have a three times greater complication rate when performed in the PD compared with in the bile duct [38]. Intraductal lithotripsy requires a specialized mother-daughter scope system or single-operator system (Spyglass system, Boston Scientific, Natick, MA, USA) to allow for direct duct visualization. There is limited discussion of these techniques in the literature. Using the mother-daughter scope system, Howell et al. [44] reported complete stone fragmentation using EHL in 3 of 6 patients, and the interim multinational registry of the Spyglass system [45] reported successful EHL of pancreatic stones in eight of 10 attempted cases (EHL was not attempted in some cases due to difficulties in placing the EHL probe in contact with the stone). Based on limited experience and technical limitations, these techniques are considered second line and are used when ESWL fails to fragment obstructive PD stones.
Results
The technical success of endoscopic removal of pancreatic stones depends on the size, number, and location of the stones [9], the presence of a ductal stricture, and whether the stone is impacted. In a series by Sherman and colleagues [39], using endoscopic techniques alone, 72% of cases had complete or partial stone removal, and 68% had symptomatic improvement. Symptomatic improvement was most evident in the group of patients with chronic relapsing pancreatitis (vs. those presenting with chronic continuous pain alone; 83% vs. 46%, respectively). When ESWL is combined with endoscopic therapy, the success of stone clearance and longer-term symptomatic relief were improved [37,40,46-49].
A meta-analysis [50] of 16 studies that included 588 patients showed that ESWL combined with pancreatic endotherapy had a significant impact on reducing pancreatic stone burden and reducing pain. A recent review showed that ESWL and endotherapy resulted in pain relief in 54-90% of patients over a follow-up of 7-173 months [24]. Dumonceau et al. [43] compared ESWL alone vs. ESWL and endotherapy; pain relapse at the end of a 2-year period was not significantly different between the two (ESWL alone, 38%; ESWL with endotherapy, 45%). The cost of ESWL alone was one-third that of ESWL with endotherapy. Patient selection with refractory disease may partly explain the much higher rates of pain relapse in this study compared with previous studies. On an intention-to-treat analysis, this study also demonstrated that the median delay between the onset of CP and persistent pain relief was 1.1 years in the treated (ESWL alone or combined therapy) group (n = 55) compared with 4 years in the untreated reference cohort (n = 42) (p < 0.001). We feel that it is helpful to remove PD stones in symptomatic patients when the stones are located in the main duct (in the head and/or body), which is more readily accessible. Ancillary ESWL will be required for more difficult stones.
PANCREATIC DUCT LEAKS/FISTULAE
PD leaks in patients with CP can occur from an upstream blowout of obstructing strictures or stones in chronic pancreatitis [51-53]. They can manifest as pancreatic ascites, internal fistulae (e.g., pseudocysts, pleural effusion to other organs), or external cutaneous fistulae. Leaks may arise from the main duct or side branches. If the leak arises from the main duct, the duct may be partially disrupted, in which case the duct retains continuity (Fig. 2A and 2B) or completely disrupted, where there is a disconnection between the upstream and downstream main duct. On the pancreatogram, the PD leak/disruption is seen as extravasation of the contrast outside the ductal structure or quick disappearance of contrast from the duct at the sites of leaks without clearance of contrast in the downstream duct (toward the head of the pancreas). When an abrupt cutoff of the PD is seen in a patient with clinical suspicion of a pancreatic leak, a disconnected duct (also known as disconnected duct syndrome or disconnected pancreatic tail) is likely, in which case viable pancreatic tissue is seen upstream on cross-sectional imaging. Diverting the pancreatic juice flow away from the fistulae and if possible bridging the pancreatic leak with transpapillary stents can successfully treat the fistulae (Fig. 2C and 2D).
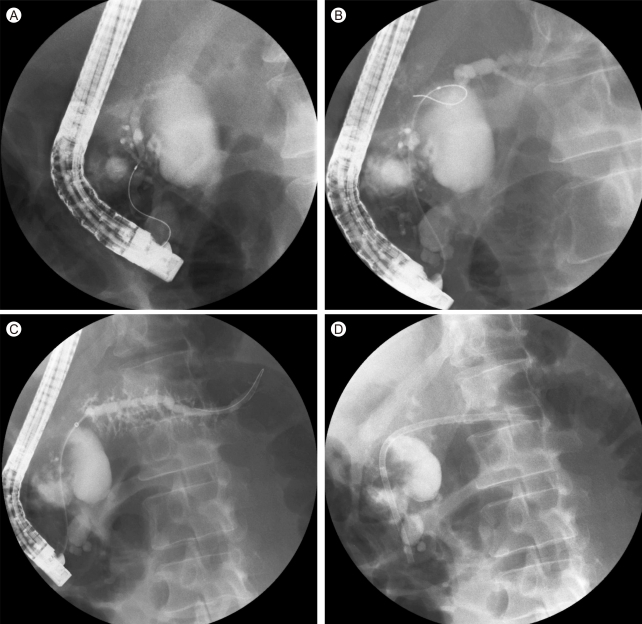
Treatment of pancreatic duct leak. (A) Leak seen from ventral duct. (B) Filling of main duct upstream of the leak. (C) Crossing the leak with a wire. (D) Placement of pancreatic stent to bridge the leak.
Results
Telford et al. [54] showed that 58% (25 of 43 patients) of pancreatic leaks resolved with pancreatic stenting with no recurrence during a 2-year follow-up period. Varadarajulu et al. [55] showed a success rate of 56% (52 of 92 patients) with multivariate analysis and showed that a partially disrupted duct and a stent bridging the disruption were associated with a successful outcome for pancreatic stenting.
Endoscopic injection of fibrin glue into the fistulous tract as an adjunct to the standard endoscopic treatment resulted in resolution of 66% of fistulae (8/12) at a median follow-up of 20.7 months [56]. However, this technique is not routinely utilized.
Complete disruption of the PD is less amenable to endoscopic therapy. In a report of 22 patients [57] with disconnected pancreatic tail syndrome treated endoscopically, 13 (59%) had either no initial response or recurrence of pancreatic leak. Although all patients in this series had acute necrotizing pancreatitis, about half had underlying CP. Endoscopic treatment of the disconnected PD syndrome is complex because the upstream-disconnected segment of the pancreas still secretes and has no communication with the duodenum. As long as this part of the pancreas remains viable, any "temporary" therapy will lead to a high recurrence rate. Thus, one endoscopic option is to drain the collection associated with the leak with transmural stents placed "permanently" or until the upstream pancreas becomes atrophic and therefore no longer secretes. To date, there have been no comparative studies of surgical, medical, and endoscopic therapy for the treatment of PD leaks.
PANCREATIC PSEUDOCYST
Pseudocysts are encapsulated collections of pancreatic juice, pure and/or containing inflammatory/necrotic debris, which are situated either outside or within the limits of the pancreas from which they arise. They complicate the course of CP in 20-40% of patients, and spontaneous resolution occurs in < 10% of cases, mostly for collections < 3 cm in diameter [58,59]. In CP, pseudocysts can occur as a result of rupture of a side branch or the main PD itself due to PD hypertension, or they may occur as a consequence of the ongoing inflammatory process. Although many pseudocysts fail to resolve spontaneously, not all require drainage. The American Society of Gastrointestinal Endoscopy recommends that pseudocyst drainage should be considered for 1) symptomatic lesions (abdominal pain, gastric outlet obstruction, early satiety, weight loss, or jaundice), 2) infected cysts, or 3) enlarging cysts [60]. If a collection develops due to an episode of acute-on-chronic pancreatitis, endoscopic drainage is usually considered after a minimum of 4-6 weeks to allow liquefaction of necrosis (thick debris does not drain well through small-diameter endoscopic stents) and for a formed wall to develop [61]. If drainage cannot be delayed due to sepsis, surgical debridement (or in selected cases, endoscopic necrosectomy with abundant lavage) is recommended [62]. Optimal management of a pseudocyst requires a multidisciplinary approach with input from surgery, gastroenterology, and interventional radiology. The technical and long-term success of endoscopic pseudocyst drainage increase for a single-compartment cyst with a mature wall and absence of necrosis.
Selection of the route (transpapillary vs. transmural) of endoscopic drainage depends on the presence of communication between the pseudocyst and pancreatic ductal system and on the size of the cyst. This information can be ascertained from any combination of imaging with EUS, magnetic resonance cholangiopancreatography (MRCP) with secretin stimulation, or ERP. Endoscopic transmural drainage is chosen if the cyst is not communicating with the duct, whereas the transpapillary route is preferred if there is communication with PD and the cyst size is relatively small (usually < 5-6 cm). If the communicating pseudocyst is particularly large, combined transpapillary and transmural drainage can be performed. Transpapillary drainage usually requires a pancreatic sphincterotomy prior to placement of the pancreatic stent. Conventional transmural drainage can be performed without EUS guidance when 1) gastric bulge/luminal impression by the cyst is present, 2) collateral blood vessels from portal hypertension are absent, and 3) the distance from the pseudocyst to the gastric/duodenal lumen on imaging studies is < 1 cm. For pseudocysts that are not amenable to such conventional endoscopic drainage, EUS-guided drainage has been shown to be equally successful, with no increased risk of complications [63]. Broad-spectrum antibiotic coverage by the intravenous route is given immediately prior to the endoscopic drainage procedure.
Technique of transmural drainage
The aim of direct cystenterostomy is to create a communication between the cyst lumen and the gastric or duodenal lumen. The first step is to puncture the gut wall at the apex of the visible bulge (Fig. 3A and 3B) using a needle knife via a duodenoscope or, if EUS is indicated, a 19-gauge fine needle aspiration (FNA) needle via a linear echoendoscope [64,65]. Once the puncture is achieved, a guide wire is advanced through the needle knife or FNA needle into the cyst cavity and looped 360° to secure positioning under fluoroscopic guidance (Fig. 3C). The newly created tract is then balloon dilated to 8-10 mm in size, or larger if necrotic material is present (Fig. 3D). This is usually followed by vigorous flow of pseudocyst fluid into the gut lumen, prior to which care should be taken to have aspiration precautions in place, with the head of the fluoroscopy table elevated and oropharyngeal suction. Two or more double pigtail stents are placed transmurally into the cyst cavity. Pre-assembled EUS-guided puncture kits are now commercially available to circumvent numerous steps of cyst entry, exchange of guide wires, and stent placement. A nasocystic drain is placed in the presence of significant debris/necrosis or infection to allow for lavage of the cyst cavity. After drainage, the size of the cyst cavity is followed via ultrasound or CT scan at 4-6-week intervals to assess resolution. Once the cyst resolves, the transmural stents can be removed. If a pancreatogram had not been obtained and ductal disease treated at the initial endoscopic procedure, this should be done at the time of stent removal.
Results
Procedural technical success ranges from 85 to 100%, and the rate of successful resolution of the cyst was approximately 90% in pooled patients from multiple series [66]. In these series, the long-term recurrence rate was 10-15%, and complications rates, primarily bleeding and perforation, were 10-34%. More recent studies [63,67,68] showed similar results with technical success > 90%, long-term cyst and symptom resolution rates ranging from 71 to 91%, and recurrence rates ranging from 16 to 18%. Baron et al. [69] reported that the rate of pseudocyst resolution was higher (92%) in patients with CP than in those with acute pancreatitis (74%), with comparable complication rates between the two groups (17% and 19%, respectively, p value not significant). These excellent results support the use of endoscopic drainage in appropriate patients, and the overall complication rates compare favorably to surgical interventions.
COEXISTING SPHINCTER OF ODDI DYSFUNCTION
Pancreatic sphincter of Oddi dysfunction (SOD) can occur primarily or secondary to deposition of protein plugs on the sphincter and extension of scarring of the pancreas [70,71]. This results in pancreatic ductal obstruction and hypertension. Pancreatic SOD is suspected with worsening symptoms and/or dilated main PD without any other structural abnormalities. This condition can be evaluated and documented by pancreatic sphincter manometry. It is primarily treated by pancreatic sphincterotomy at the major papilla and placement of temporary pancreatic stent for prophylaxis against post-ERCP pancreatitis. Okolo et al. [72] reported that in 55 patients (40 with chronic pancreatitis) who underwent pancreatic sphincterotomy for manometrically documented pancreatic SOD, 60% had improved pain scores at a median follow-up of 16 months. In another study, Gabbrielli et al. [23] reported that 64% of CP patients with a dilated PD, of whom 45.5% (5 of 11) had no pancreatic stones or stricture, had absence of pain at a mean follow-up of 6.5 years with pancreatic sphincterotomy alone. These data and our experience show that pancreatic sphincterotomy can be used as sole therapy in cases of documented pancreatic sphincter hypertension coexisting with CP. In our experience, we have found that at least 40% of patients with CP have associated sphincter of Oddi hypertension. However, it is unclear whether this contributes to CP or is the result of CP. There have been no reports regarding the outcomes of pancreatic sphincterotomy in patients with elevated sphincter of Oddi pressure in the setting of nondilated PD. We are currently conducting a prospective trial to study the outcomes of sphincterotomy in patients with pancreatic SOD with and without CP.
COEXISTING PANCREAS DIVISUM
Pancreas divisum is the most common congenital variant of pancreatic ductal anatomy and occurs when the dorsal and ventral PDs fail to fuse during the second month of gestation. With duct nonunion, the major portion of the pancreatic exocrine juice drains into the duodenum via the dorsal duct and minor papilla. It has been proposed that relative obstruction of pancreatic exocrine juice flow through the minor papilla could result in pancreatic-type abdominal pain, acute pancreatitis, or CP in a subpopulation of pancreas divisum patients [73]. Endoscopic attempts to decompress the dorsal duct in symptomatic pancreas divisum patients have been performed primarily by dilation, stent insertion, and/or minor papilla sphincterotomy. The reported symptom improvement following endoscopic therapy for pancreas divisum in the setting of CP is much lower than for the recurrent acute pancreatitis. In 52 patients treated (11 with CP) by minor papilla sphincterotomy, Lehman et al. [74] reported that 27% of the CP group benefited compared with 76.5% of the acute recurrent pancreatitis group followed up for periods between 6 months and 1.5 years. A longer-term study (median follow-up of 43 months) of 113 pancreas divisum patients (22 had CP) treated by minor papilla therapy was reported by Borak et al. [75]. Primary and secondary success rates for CP were 18.2% and 45.5% vs. 53.2% and 71% in the acute and recurrent pancreatitis groups, respectively.
These data show that minor papilla therapy of pancreas divisum patients with CP is less effective than the same therapy in patients with acute recurrent pancreatitis. However, endoscopic therapy can still be offered to patients with disabling symptoms. It should be appreciated that the endoscopic approach to ductal disease in patients with pancreas divisum is the same as in nondivisum patients.
ENDOTHERAPY ON ADJACENT STRUCTURES
Distal common bile duct stricture
Common bile duct stricture in CP can be malignant or from benign disease. It is important to exclude malignancy with a CT scan of the abdomen with pancreatic protocol and EUS with or without FNA prior to endoscopic treatment if there is clinical suspicion. Once the biliary stricture is determined to be benign, it is important to identify correctable causes, such as a pseudocyst causing extrinsic compression on the bile duct or pancreatic inflammation/edema in the setting of acute exacerbation of CP. The precise incidence of benign biliary strictures (BBSs) secondary to fibrosis and calcification within the pancreatic head is unknown and varies from 3 to 46% [76]. This type of BBS is irreversible without intervention and can result in jaundice, cholangitis, and progressive secondary biliary cirrhosis. Indications for biliary decompression in CP include symptoms (e.g., jaundice, cholangitis), biliary cirrhosis, common bile duct stones, progression of biliary stricture, and persistent asymptomatic elevation of alkaline phosphatase and/or bilirubin for > 1 month (a threshold twice the upper limit of normal values is often chosen for alkaline phosphatase) [77]. Hammel et al. [78] reported regression of hepatic fibrosis when the CBD was surgically drained. Although traditionally, BBSs in CP are treated by surgery with good long-term success, there is significant surgical morbidity in frequently debilitated patients with alcoholic CP and associated liver disease. Deviere et al. [79] were first to report the use of endoscopic treatment of the BBS in CP with endoscopic sphincterotomy, followed by insertion of one or two 10 Fr biliary stents with as-needed stent changes. Although technical success and short-term resolution of cholestasis and cholangitis was 100%, long-term (mean, 14 months; range, 4 to 72) stricture resolution was much less satisfactory, occurring at a rate of 12%. Another study also indicated low stricture resolution rates of only 10-38% over a mean follow up of 18-58 months [25]. Subsequent studies involving serial placement of multiple simultaneous biliary stents to achieve gradual dilation of the BBS improved outcomes, with a long-term resolution rate of BBSs with multiple plastic stents ranging from 44 to 92% over a 12 to 48-month follow-up period [80-82].
SEMSs (uncovered and partially covered) designed for permanent placement were evaluated for BBSs in the setting of CP. Again, technical success and initial reversal of cholestasis and cholangitis were 100%, although at long-term follow-up ranging from 22 to 50 months, the stents were occluded in 10-62% of cases. Stent patency rate dropped from 100% at 12 months to 40% at 24 months and 37.5% at 30 months [83-85]. In such patients, subsequent options are to refer for surgery or repeat endoscopic procedures for stent-in-stent placement.
In an attempt to decrease the number of serial endoscopic procedures and avoid the disadvantage of clogged permanent stents. Kaheleh et al. [86] reported stricture resolution after a median stent interval of 4 months (range, 1 to 28) with a partially covered metal stent and no recurrent symptoms during a 12-month (range, 3 to 26) follow-up period after stent removal in 17 of 22 (77%) patients with CP. The same group had less success with fully covered stents. Eleven of 19 patients (58%) stented for a median of 3.3 months with a fully covered metal stent showed resolution of their stricture and no recurrent symptoms after stent removal during a 3.8-month follow-up period [87].
Briefly, in selected patients who are high-risk surgical candidates and those who wish to undergo endoscopic treatment for BBSs, endoscopic stenting with multiple plastic stents and/or self-expandable removable metal stents appears to be a potentially useful second-line therapy. However, more data on long-term outcomes, preferably in randomized trials in large numbers of patients, will be necessary before this therapy can be advocated as routine.
EUS-guided celiac plexus block/neurolysis
One of the mechanisms of pain related to CP is peripancreatic and celiac neuronal inflammation. There has been long-standing interest in decreasing neuronal inflammation with steroids and decreasing the perception of pain with anesthetic nerve block or neurolysis with alcohol. Neurolysis is usually reserved for malignant diseases because of concerns regarding the long-term effects of alcohol injection, including retroperitoneal fibrosis. Percutaneous (via lumbosacral muscles) or surgical approaches were used previously; however, transgastric EUS-guided celiac plexus block (EUS-CPB) has gained favor in the last decade due to the high success rate, lower complication rates, and simplicity of performing the procedure.
Technique
In this technique, a curvilinear array echoendoscope is used to identify the origin of the celiac trunk from the abdominal aorta. A recent report [88] also detailed the successful use of a prototype forward-viewing echoendoscope for performing celiac plexus neurolysis. The Mayo group showed that celiac ganglia can be directly visualized on EUS in 81% of patients [89] and that performing celiac ganglia block was technically feasible [90]. However, to date, there have been no studies comparing celiac ganglia block vs. celiac plexus block. Sakamoto et al. [91] recently showed that EUS-guided broad plexus neurolysis over the superior mesenteric artery using a 25-gauge needle gave better pain relief in pancreatic cancer patients than did conventional celiac plexus neurolysis. Whether this technique is applicable in cases of chronic pancreatitis using broad plexus block is still unknown.
For celiac plexus or ganglia block, a 22- or 19-gauge EUS FNA needle is used for injection into the celiac region or, if feasible, directly in to the celiac ganglion. Once the FNA needle is advanced to the appropriate location, aspiration is first performed to make certain a vascular puncture has not occurred. Bupivacaine is first injected, followed by triamcinolone. Injection can be performed on one or both sides of the celiac trunk. In a prospective randomized trial, LeBlanc et al. [92] showed no difference in technical success, symptom response, or complication rates between one and two sites of injection during the same EUS-CPB session. Owing to the risk of hypotension, patients should be closely monitored for 2-4 hours after the procedure.
Results
In a retrospective series [93] and two prospective series [94,95], the overall success rate for EUS-CPB was 95%. The short-term pain improvement rate was 50-55%, and long-term pain relief at 12 weeks was 26%, whereas that at 24 weeks was 10%. Younger patients (< 45 years of age) and those with previous pancreatic surgery for CP were unlikely to have pain relief with EUS-CPB [94]. In a recent meta-analysis of 221 pooled patients from six studies, Kaufman et al. [96] found that the rate of short-term relief of abdominal pain from CP was 51.46%. Another meta-analysis of 376 patients from nine studies conducted by Puli et al. [97], which included both celiac plexus block and neurolysis for chronic pancreatitis, showed 59.45% short-term pain relief. Celiac ganglion injection under endosonographic guidance yielded pain improvement in five of 13 (38%) patients with CP when steroids were used vs. four of five (80%) patients receiving alcohol [90]. To date, there have been no studies comparing celiac plexus injection to sham controls [98], and there have been no head-to-head studies comparing neurolysis to nerve block in patients with chronic pancreatitis. Due to a lack of definite data, the relatively low response rates, and the requirement for repeat procedures, given the short duration of pain relief, EUS-CPB should be considered a temporizing measure reserved for cases in which oral analgesia is ineffective or in patients intolerant to medication side effects pending a more definitive intervention.
CONCLUSIONS
CP is a debilitating disease with pain as its major presentation. The cause of pain is multifactorial and is thought to arise from several complications, such as ductal strictures, ductal stones, pseudocysts, and pancreatic cancer, which contribute to significant morbidity and mortality. A multidisciplinary approach on a case-by-case basis involving medical, endoscopic, and surgical management is the ideal approach for treatment of CP. Although two randomized studies have shown that surgical management is more durable for the treatment of pain in a select group of patients with PD strictures/stones in the head and upstream dilation, it may not always be a feasible option due to patient comorbidities or preferences. Endotherapy can be performed with various combinations of pancreatic sphincterotomy, stent placement, stricture dilation, ESWL, stone removal, pseudocyst drainage, and EUS-guided access and therapy. Endoscopic management has shown reasonable success in select patient populations, although the true rate of success is difficult to determine due to the retrospective nature of the majority of studies as well as to the variability in natural history and the multifactorial nature of pain in CP. Further randomized comparative controlled trials are warranted to define the role of endoscopic treatment of CP. Further refinements in endoscopic equipment dedicated to the pancreas and advancements in technology may help to improve the outcomes of endoscopic treatment.
Notes
No potential conflict of interest relevant to this article was reported.
