Clinical Significance of Observation without Repeated Radioiodine Therapy in Differentiated Thyroid Carcinoma Patients with Positive Surveillance Whole-Body Scans and Negative Thyroglobulin
Article information
Abstract
Background/Aims
Currently, there is no consensus on the necessity of repeated radioiodine therapy (RAI) in patients who show iodine uptake in the thyroid bed on a diagnostic whole-body scan (DxWBS) despite undetectable thyroglobulin (Tg) levels after remnant ablation. The present study investigated the clinical outcomes of scan-positive, Tg-negative patients (WBS+Tg-) who did or did not receive additional RAI.
Methods
We retrospectively reviewed 389 differentiated thyroid carcinoma patients who underwent a total thyroidectomy and received high-dose RAI from January 2003 through December 2005. The patients were classified according to surveillance DxWBS findings and TSH-stimulated Tg levels 6 to 12 months after the initial RAI.
Results
Forty-four of the 389 patients (11.3%) showed thyroid bed uptake on a DxWBS despite negative Tg levels (WBS+Tg-). There was no difference in clinical and pathological parameters between WBS+Tg- and WBS-Tg- patients, except for an increased frequency of thyroiditis in the WBS+Tg- group. Among the 44 WBS+Tg- patients, 27 subjects were treated with additional RAI; 25 subjects showed no uptake in subsequent DxWBS. Two patients were evaluated only by ultrasonography (US) and displayed no persistent/recurrent disease. The other 17 patients received no further RAI; Eight patients and two patients showed no uptake and persistent uptake, respectively, on subsequent DxWBS. Six patients presented negative subsequent US findings, and one was lost to follow-up. Over the course of 53.2 ± 10.1 months, recurrence/persistence was suspicious in two patients in the treatment group.
Conclusions
There were no remarkable differences in clinical outcomes between observation and treatment groups of WBS+Tg- patients. Observation without repeated RAI may be an alternative management option for WBS+Tg- patients.
INTRODUCTION
The detection of papillary thyroid carcinoma (PTC) has been increasing globally due to the widespread use of sensitive diagnostic tools such as high-resolution ultrasonography (US). In addition to measurements of serum thyroglobulin (Tg), whole-body scans (WBS) with radioiodine has been considered the main tool for detecting persistent or recurrent disease during follow-up of differentiated thyroid carcinoma (DTC). However, recent published guidelines, as well as several previous reports, discourage the use of diagnostic WBS (DxWBS) as a follow-up method, especially for low-risk patients with DTC because of its low sensitivity and the lack of additional information it provides as compared with Tg measurements [1-5].
A few recent reports suggest that lesions detected only by diagnostic or therapeutic WBS without detectable Tg levels could be recurrent/persistent disease or a clinically significant lesion. This information has shifted the focus back on the importance of WBS [6,7]. On the other hand, previous findings have considered thyroid bed uptake in WBS after total thyroidectomy and remnant ablation to be clinically insignificant and evidence of remnant ablation failure without regard to persistence/recurrent lesions in DTC [2].
The present study was performed to evaluate the clinical characteristics, prognosis, and possible management plan of patients with positive WBS who demonstrate thyroid bed uptake despite undetectable Tg levels after high-dose radioiodine therapy (RAI).
METHODS
Patients
A retrospective review was conducted on 699 patients who had undergone total thyroidectomy for DTC and received RAI from January 2003 through December 2005 at our institution. Patients were excluded if they did not have high-dose RAI, follow-up Tg measurements were not performed on a regular basis, thyroid stimulating hormone (TSH) levels were < 30 IU/L at the time of radioiodine scan, or they were positive for anti-Tg antibody (Ab) (> 70 IU/mL) with Tg levels < 1 ng/mL. A total of 389 patients with DTC were included.
Radioiodine whole-body scan and remnant ablation
Thyroid hormone (T4) was withdrawn and replaced with 25 µg of triiodothyronine (T3) every 12 hours for 2 weeks, and T3 was stopped for at least 2 weeks to stimulate TSH > 30 IU/L, according to the institutional protocols. The initial treatment dose was determined by diagnostic 123I-WBS performed prior to high-dose RAI and post-operative histologic findings. The patients followed a low-iodine diet for at least 2 weeks before the diagnostic 123I-WBS. After oral administration of approximately 111 MBq (3 mCi) of 123I, scans were obtained using a high-resolution collimator set at 159 keV with a 15% energy window using the E-CAM dual detector system (Siemens, Erlangen, Germany). In general, 3700 MBq (100 mCi) was administered for patients with uptake limited to the thyroid bed, 5500 MBq (150 mCi) when uptake was suspected in the cervical region, and 7400 MBq (200 mCi) for suspicious distant metastasis. All patients received TSH-suppressive doses of L-thyroxine for at least 2 years.
Tg, anti-Tg antibody, anti-TPO antibody, and TSH measurement
Serum Tg levels were measured using an immunoradiometric assay (IRMA) kit (CIS Bio International, Cedex, France). The intra-assay coefficient of variation (CV) was 7.7%, 2.6%, and 1.4% at 1.22, 43.8, and 116.0 ng/mL, respectively. The inter-assay CV was 16.7%, 3.1%, and 2.0% at 0.8, 43.0, and 111.0 ng/mL, respectively, and the functional sensitivity was 0.7 ng/mL. Anti-Tg Ab and anti-thyroid peroxidase Ab were measured by a competitive radioimmunoassay (RIA) kit (ZenTech, Angleur, Belgium), and the given normal range was < 70 IU/mL and 50 IU/mL, respectively. TSH levels were measured by an IRMA kit (Beckman Coulter, Prague, Czech Republic), with a detection limit of 0.025 mIU/L. The intra- and inter-assay CV were 3.7% and 5.7%, respectively. Follow-up management of patients with DTC after surgery included physical examination, serum TSH, free T4, Tg, and anti-Tg Ab measurements with or without calcium and phosphorus tests. These tests were repeated at 3-month or shorter intervals and continued for the first 2 years after surgery.
Follow-up management
All patients underwent an initial follow-up examination with clinical evaluation, 123I DxWBS, and Tg after L-thyroxine withdrawal with or without US 6 to 12 months after RAI. DxWBS results were visually interpreted by two experienced nuclear medicine physicians who reached a consensus. Strong radioiodine uptake in the thyroid bed on initial post-therapeutic whole-body scan (TxWBS) was interpreted as a normal thyroid remnant.
Stimulated Tg levels above 5 to 10 ng/mL were considered to indicate residual or recurrent tumor, regardless of the WBS finding. Stimulated Tg < 1 ng/mL, negative DxWBS, and normal US, when performed, were considered as lack of evidence for recurrent/persistent disease. Anterior neck uptake in the thyroid bed without abnormal radioiodine uptake elsewhere on WBS, despite negative Tg levels < 1 ng/mL, was the target. WBS+Tg- patients were managed according to each physician's clinical decision and not according to a specific common treatment protocol. The treatment group who received a second bout of RAI therapy was followed up with Tg and DxWBS and/or US within 1 year. The observation group had Tg measurements and US and/or DxWBS performed within 6 to 12 months. Other imaging studies such as computed tomography (CT), magnetic resonance imaging (MRI), and F-18 fluorodeoxyglucose positron emission tomography/CT (FDG PET/CT) were performed when metastasis outside the neck was clinically suspected. This study was approved by our institutional review board.
Statistical analysis
Statistical analysis of the categorical variables was conducted using a χ2 test or Fisher's exact test. Comparisons between continuous variables were performed with an independent t test. A p value < 0.05 was considered statistically significant. All the analyses were performed using SPSS version 12.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Among 389 patients with DTC, 44 (11.3%) showed thyroid bed uptake on DxWBS but negative thyroglobulin levels (WBS+Tg-) 6 to 12 months after initial remnant ablation with high-dose RAI. The success rate of initial high-dose remnant ablation was 64.8% (252/389), which was the number of WBS-Tg- subjects. The number of cases according to the four different combinations of WBS and serum Tg level results is presented in Fig. 1. There were no differences in clinical and pathological parameters between the WBS+Tg- group and the WBS-Tg- group except for thyroiditis, which was more frequently observed in WBS+Tg- patients (27.3% vs. 11.5%, p = 0.005) (Table 1). Other clinical and histological characteristics and factors of prognostic relevance are summarized in Table 1. Additionally, there was no significant difference between WBS+Tg- and WBS-Tg- groups in the time from high-dose remnant ablation to the first follow-up DxWBS, although there was a trend toward a slightly longer duration in the WBS-Tg- group (WBS+Tg- vs. WBS-Tg-, 7.4 ± 2.1 months vs. 8.1 ± 3.7 months, p = 0.073). Of the 44 WBS+Tg- patients, 27 were treated with repeated high-dose RAI therapy (100 or 150 mCi) in addition to the first RAI (Table 2). The other 17 patients were observed without further RAI. The treatment group (n = 27) and observation only group (n = 17) were allocated as determined solely by each physicians' clinical decision without randomization or blinding. However, the two groups showed no significant differences in clinical and post-operative pathology risk parameters (data not shown).

Classification of study subjects according to the first diagnostic whole body scan (WBS) and thyroid stimulating hormone (TSH)-stimulated serum thyroglobulin (Tg) results 6-12 months after high-dose radioiodine remnant ablation. Negative Tg is defined as serum Tg levels < 1 ng/mL.
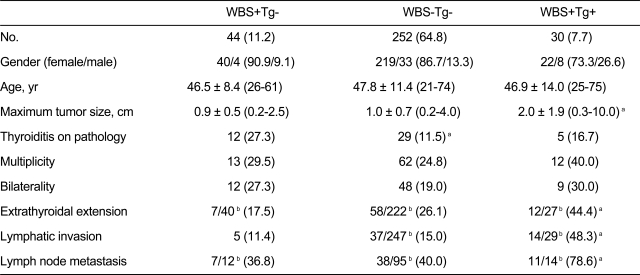
Comparison of clinical and histopathological characteristics among WBS+Tg-, WBS-Tg- and WBS+Tg+ differentiated thyroid cancers
All treated subjects displayed thyroid bed uptake in therapeutic WBS after repeat RAI therapy, with the exception of three patients who showed no definite uptake either inside or outside the thyroid bed. Of the 27 treated subjects, 25 showed no uptake in the thyroid bed on the next DxWBS. The other two patients were checked only by US, which revealed no evidence of remnant or recurrent disease (Table 2). Of the 17 patients in the observation only group, eight showed no uptake in the thyroid bed on subsequent DxWBS without further treatment. Among the other nine patients, six patients demonstrated negative US findings, and follow-up DxWBS was not performed. Two patients showed persistent uptake on follow-up DxWBS. One patient showed a small hypoechoic lesion (3.1 × 3.8 mm) in the thyroid bed, which was regressed and then eventually resolved on annual US examination, and the other patient showed negative US findings (Table 2). One patient was lost to follow-up. During the 53.2 ± 10.1-month follow-up period, all but two suspicious cases out of the 44 WBS+Tg- subjects were negative for recurrence. A case from the treated group showed mildly increased Tg levels (1.16 ng/mL) off L-thyroxine with no uptake on follow-up DxWBS. Another treated case showed a < 5-mm-diameter hypoechoic lesion in the thyroid bed in two consecutive US findings with no interval change. Both cases were recurrence free if a Tg level of 2 ng/mL was used as a cut-off, and the US detected a post-operative granuloma.
To investigate the possible effects of remnant thyroid lesions at the time of initial RAI therapy on subsequent DxWBS thyroid bed uptake 1 year after RAI, we evaluated Tg levels and the number of patients with Tg > 1 ng/mL and Tg > 5 ng/mL at the time of RAI therapy in both WBS+T- and WBS-Tg- groups (Table 3). No significant differences were noted in Tg levels or in the number of patients with Tg levels > 1 ng/mL or > 5 ng/mL between the WBS+Tg- group and the WBS-Tg- group.
DISCUSSION
Owing to the high sensitivity and specificity of US, the value and role of DxWBS in DTC follow-up has decreased. Additionally, the use of DxWBS has been discouraged in the follow-up of low-risk differentiated thyroid carcinoma. One recent report supported the utility of pre-ablation radioiodine scan, although this view remains contentious [8-10]. In addition, the occasional remnant uptake in the thyroid bed after high-dose remnant ablation is considered to be a kind of failure of initial remnant ablation or a non-specific finding [11]. However, no clear explanation is available for a WBS-positive lesion with negative Tg.
In the present study, the clinical characteristics of WBS+Tg- patients were not different from those of WBS-Tg- patients. Previous reports ignored the clinical significance of radioiodine uptake in the thyroid bed after remnant ablation because of low thyroglobulin levels, which is considered a good prognostic marker [2,11-13]. However, a few recently published reports emphasized the importance of WBS [6-8]. One report concerning positive post-therapeutic WBS uptake outside the thyroid bed and negative thyroglobulin levels suggested metastatic lesions rather than false-positive image findings [7]. Another report demonstrated that many patients were positive for thyroid bed uptake on DxWBS and negative for Tg levels after high-dose RAI. These patients responded well to additional high-dose RAI therapy, discouraging the use of a negative Tg value as an adequate solitary marker for the presence and treatment of small cervical remnants [6].
The current findings also suggest that thyroid bed uptake on DxWBS after high-dose RAI could be easily treated with subsequent additional radioiodine therapy. However, little evidence supports the view that this management plan actually improves survival or decreases recurrence in such low-risk patients with undetectable Tg levels. Pacini et al. reported persistence of minor uptake in the thyroid bed in a minority of patients in whom the previous ablative dose of 131I was not totally effective. In nearly one-third of these cases, spontaneous disappearance of thyroid bed uptake at the follow-up DxWBS was observed without retreatment with radioiodine, suggesting no influence on final outcome [2]. In the present study, both the initial clinical parameters and last follow-up outcomes, including recurrence rates, were similar between treatment and observation groups (Tables 1 and 2). These results suggest that uptake in the thyroid bed concurrent with negative Tg does not require immediate treatment. When considering reports about the hazards of RAI therapy in DTC patients, such as second primary malignancy [14-16] and chronic sialadenitis [17,18], additional high-dose RAI therapy aimed at decreasing remnant uptake in WBS may do more harm than good. Moreover, the difficulties the patient endures during radioiodine therapy, including the tedious low-iodine diet, long period of hypothyroidism, and acute radiation-induced side effects, must also be taken into consideration. Moreover, as US can detect suspicious lesions of very small volume in the neck area, it would be reasonable to use US for monitoring low-risk DTC patients showing WBS uptake only in the thyroid bed. This policy is already reflected in recent management guidelines [4,5].
The cause of uptake in the thyroid bed on 123I DxWBS following total thyroidectomy and high-dose RAI is unknown. It is generally believed that the remaining normal thyroid tissue persists despite high-dose RAI therapy and that the previous radioiodine dose is not totally effective in these patients [2]. On the other hand, according to some reports, a small tumor mass detected by WBS might account for negative Tg levels, and after repeated radioiodine therapy, viable cancer cells diminish and eventually disappear on WBS [7,19]. The fact that microscopic thyroiditis was more frequently observed on WBS+Tg- on post-operative pathology (Table 1) led us to retrospectively review anti-Tg Ab and anti-TPO Ab status. Although anti-TPO Ab measurement was not performed pre- and post-operatively in most of the cases, anti-Tg Ab is a routine laboratory parameter in our thyroid nodule clinic along with Tg. Additionally, pre- and post-operative follow-up measurements were available in the majority of cases.
Serum anti-Tg Ab levels in WBS+Tg- patients tended to be higher than that in WBS-Tg- patients within the normal range (data not shown), suggesting that remaining autoimmune processes or some sort of mild inflammatory condition might be involved in thyroid bed uptake on DxWBS. However, it is not yet fully understood whether increased serum anti-Tg Ab levels reflect remnant normal thyroid tissue.
The present study did not detect differences in thyroglobulin levels immediately at the time of initial high-dose RAI, which reflects remnant thyroid tissue after total thyroidectomy. These results indirectly suggest that the initial volume of remnant thyroid tissue has no effect on thyroid bed uptake in subsequent DxWBS. However, as undetectable serum Tg levels always signify no minimal tumor burden in patients who have already been treated with RAI [20], US should be used to evaluate metastatic lymph nodes in such patients.
Another distinguishing feature of this study was the use of 123I for diagnostic WBS instead of 131I for the first and the second follow-up 6 to 12 months after RAI. It was reported that 123I diagnostic WBS is comparable to post-therapeutic scans performed immediately after high-dose RAI therapy, with a concordance rate exceeding 95% [21,22]. The presently observed thyroid bed uptake on 123I DxWBS was comparable to the post-therapeutic WBS, bolstering confidence in assessing the risk stratification of DTC patients using 123I DxWBS.
The present study has several limitations. First, the study was retrospective, and the patients who received additional RAI therapy were not randomly allocated, but instead distributed according to each clinician's judgment. The physician may have decided to repeat radioiodine therapy in higher-risk patients, even though the clinical characteristics were not significantly different. Second, the mean follow-up duration was 53 months, which is too short to fully evaluate recurrence in the low-risk DTC group. Finally, although experienced nuclear medicine physicians reviewed the WBS, the percentage uptake of radioiodine in the thyroid bed was not routinely measured, and this may have decreased the accuracy of WBS.
The results support a wait-and-watch approach without further high-dose radioiodine treatment as a feasible option for WBS+Tg- patients, especially those that are low risk. The present findings should prove useful for thyroid cancer clinics still performing a DxWBS-based radioactive iodine therapy. However, a randomized trial with a greater number of patients is necessary to determine the long-term significance of WBS thyroid bed uptake despite low thyroglobulin levels in DTC management.
Notes
No potential conflict of interest relevant to this article was reported.

