Usefulness of Peak Systolic Strain Measurement by Automated Function Imaging in the Prediction of Coronary Perfusion in Patients with Acute Myocardial Infarction
Article information
Abstract
Background/Aims
The usefulness of global longitudinal peak systolic strain (GLPSS) measurement by automated function imaging (AFI) in the prediction of perfusion status of infarct-related artery (IRA) before percutaneous coronary intervention (PCI) was evaluated.
Methods
Sixty-nine patients with acute myocardial infarction (AMI) who underwent successful primary PCI were divided into two groups; the patients with occlusion of IRA (Group I, 41 patients, 63.0 ± 14.9 years of age, 31 males) versus the patients with patent IRA (Group II, 28 patients, 63.8 ± 11.2 years of age, 15 males). GLPSS by AFI and wall-motion score index (WMSI) were analyzed in both groups.
Results
GLPSS was significantly decreased in Group I compared with Group II (-11.2 ± 3.7 vs. -14.1 ± 4.7%, p = 0.005), but WMSI (1.49 ± 0.28 vs. 1.35 ± 0.32, p = 0.062) did not differ between groups. GLPSS of infarct segments was significantly lower (-3.7 ± 5.4 vs. -11.4 ± 4.8%, p < 0.001), and WMSI of infarct segments was significantly higher (2.13 ± 0.57 vs. 1.66 ± 0.57, p = 0.001) in Group I compared with Group II. By receiver operation curve analysis, the area under the curve to predict IRA occlusion was 0.850 in GLPSS of infarct segments and 0.719 in WMSI of infarct segments. The optimal cut-off value to predict IRA occlusion was -9.4% in GLPSS of infarct segments (sensitivity, 85.4%; specificity, 67.9%) and 1.68 in WMSI of infarct segments (sensitivity, 78.0%; specificity, 60.7%).
Conclusions
The present study suggested that GLPSS measured by AFI is a more sensitive predictor of IRA occlusion than is WMSI before PCI. Routine measurement of GLPSS by AFI can be a very useful tool in risk stratification of AMI.
INTRODUCTION
The perfusion status of infarct-related artery (IRA) is a significant predictor of infarct size and clinical outcome and is thus important in the risk stratification of acute myocardial infarction (AMI) [1-4]. Previous studies have shown that patients with spontaneous reperfusion of IRA show better clinical outcomes and have smaller infarcts [5-9].
Strain and strain-rate imaging by a 2-dimensional (2D) speckle tracking method is a novel echocardiographic modality to evaluate regional and global myocardial function, and is relatively free from the angle dependency and frame-rate limitation of tissue Doppler imaging (TDI) [10-13]. Automated function imaging (AFI) is a newly introduced computerized 2D-based strain measurement tool to highlight potential wall-motion abnormalities. 2D strain analysis allows more accurate evaluation of regional and global myocardial function, infarct size, viability of the infarcted myocardium, and subtle changes related to myocardial ischemia than do conventional 2D echocardiographic methods, such as wall-motion score (WMS) or wall-motion score index (WMSI), in patients with AMI. Therefore, 2D strain analysis provides useful information not only in the diagnosis of AMI, but also in the risk stratification and prognostication of future clinical outcomes.
Although attempts have been made to detect coronary reperfusion before percutaneous coronary intervention (PCI) by analyzing myocardial integrated backscatter [14] and contrast echocardiography [15], data are lacking on the relationship between the perfusion status of IRA before PCI and global longitudinal peak systolic strain (GLPSS) as measured by this novel 2D strain analysis in patients with AMI. Therefore, the aim of this study was to investigate the usefulness of GLPSS measurement by AFI compared with WMSI in predicting coronary perfusion in patients with AMI.
METHODS
Study subjects
From October 2006 to September 2007, a total of 76 patients with AMI who underwent successful primary PCI within 12 hours from the onset of AMI for single vessel disease and who did not meet the exclusion criteria were initially enrolled. However, six patients with poor image quality and one patient who withdrew informed consent after image acquisition were excluded. Therefore, a total of 69 patients were included for analysis.
The study protocol was approved by the Institutional Review Board, and informed consent was obtained from each patient. Exclusion criteria were 1) a history of previous myocardial infarction (MI) or PCI, 2) significant multi-vessel disease, 3) cardiogenic shock at presentation, 4) thrombolytic therapy before admission, 5) atrial fibrillation/flutter or significant ventricular arrhythmias, 6) significant valvular steno-insufficiency, and 7) patients who could not perform the echocardiographic examination before PCI or within 1 hour after primary PCI.
Echocardiographic examination
Echocardiographic examinations were performed before PCI or within 1 hour following successful PCI to minimize the impacts of PCI. Routine echocardiographic examinations were performed by the recommendation of the current guidelines. Echocardiographic images from the various echocardiographic windows, including three apical views, were obtained using digital ultrasound equipment (Vivid 7, GE Vingmed Ultrasound, Horten, Norway). Digital cine loops were obtained for subsequent off-line analysis. All data were analyzed by a computerized off-line software package (EchoPAC PC 6.0.0, GE Medical Systems, Horten, Norway).
Regional wall motion of the left ventricle (LV) was evaluated by calculating WMS or WMSI of the LV as suggested by the American Society of Echocardiography [16]. A numeric scoring system was adopted for each segment based on the contractile status (normal, 1; hypokinesia, 2; akinesia, 3; dyskinesia, 4; aneurysm, 5). The WMSI was calculated by dividing the sum of the WMS by the number of visualized segments, and the WMSI of the IRA was calculated by dividing the sum of the WMS of the IRA segments by the number of visualized segments.
Measurement of strain by AFI
GLPSS of the LV was measured by AFI at a frame rate of 56.2 ± 11.7 frames/sec. After selecting the optimal 2D image, the timing of aortic valve closure was derived from the pulse wave Doppler of the aortic valve, and the three-point click method in three apical planes (apical four-chamber, two-chamber, and long axis view) was used. The LV in each apical view was divided into three levels (basal, mid, and apical), and each level was subdivided into two segments (septal and lateral); thus, the LV was divided into six segments for each apical plane. Two points placed at the base along the mitral valve annulus and one at the apex triggered the automated process. AFI non-invasively tracked and analyzed GLPSS based on the 2D speckle tracking method and displayed the combined results of GLPSS of the three planes in a single bull's eye summary. The mean value of GLPSS was calculated by dividing the sum of the GLPSS of each segment by 18, and the mean GLPSS of the IRA was calculated by dividing the sum of the GLPSS of the IRA segments by the number of IRA segments. A representative example of the measurement of the GLPSS by AFI is shown in Fig. 1.

Representative example of the measurement of peak systolic strain by automated function image in a patient with an acute anterior wall myocardial infarction caused by the total occlusion of the mid-portion of the left anterior descending coronary artery. Peak systolic strain of anferior and septal wall was decreased in 4-chamber (A), 3-chamber (B), and 2-chamber (C). The decreasema of peak systolic strain of left anterin descending coronary antery territory was shown on Bull's eye view (D). ANT, anterior; LAT, lateral; POST, posterior; INF, inferior; SEPT, septal.
Assessment of coronary perfusion
Coronary angiography and PCI were done as soon as possible after confirming a diagnosis of AMI. The IRA was determined by comparing electrocardiography, echocardiography, and coronary angiography. Coronary perfusion of the IRA was assessed using thrombolysis in myocardial infarction (TIMI) flow grading; TIMI 0, total occlusion; TIMI 1, slow and incomplete opacification of the artery portion after the lesion; TIMI 2, slow but complete opacification; and TIMI 3, normal perfusion. TIMI grade 0 or 1 before PCI was considered occlusion of the IRA, and TIMI 2 or 3 was considered patent IRA.
Statistical analysis
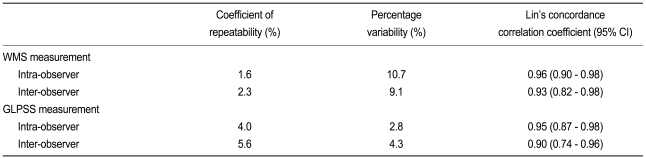
The SPSS version 13.0 (SPSS Inc., Chicago, IL, USA) and MedCalc software version 9.0 (MedCalc, Mariakerke, Belgium) were used for statistical analyses. The values were expressed as the mean value ± standard deviation. The comparisons between the groups were conducted by student's t test for continuous variables and the chi-square test for categorical variables. To identify the optimal cutoff value, receiver operation characteristic (ROC) curve analysis was conducted. Reproducibility of the measurement of the GLPSS by AFI was examined in 10 randomly selected cases by two independent observers (JSC and KHK). Intra- and inter-observer variability were estimated by measuring the percentage variability (absolute differences between the two measurements divided by the mean of the two measurements), the coefficient of repeatability using the Bland-Altman statistics, and the Lin's concordance correlation coefficient. A p value < 0.05 was considered statistically significant.
RESULTS
Baseline characteristics
The patients were divided into two groups according to results of coronary angiography: patients with occlusion of IRA (Group I, 41 patients, 63.0 ± 14.9 years of age, 31 males) and the patients with patent IRA (Group II, 28 patients, 63.8 ± 11.2 years of age, 15 males).
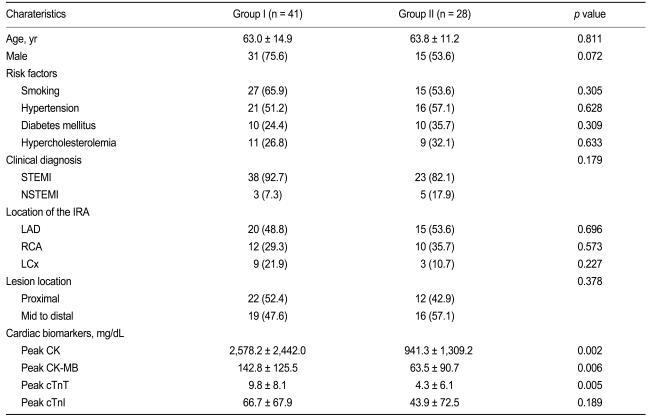
The baseline characteristics are summarized in Table 1. The clinical diagnosis was acute ST segment elevation MI (STEMI) in 61 patients (88.4%) and non-ST segment elevation MI (NSTEMI) in eight patients (11.6%). No significant differences in clinical characteristics including age, sex, risk factors, and the location of the IRA were found between groups. The peak level of creatinine kinase, MB fraction of creatinine kinase, and cardiac specific troponin-T were significantly higher in Group I than in Group II.
Echocardiographic findings
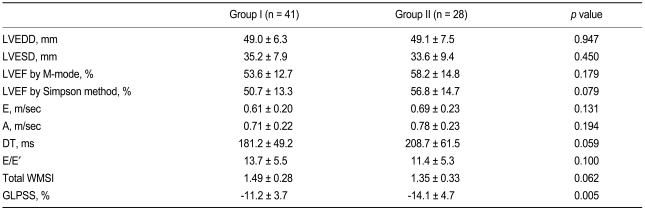
Echocardiographic findings are summarized in Table 2. The total WMSI did not differ between the groups (1.49 ± 0.28 vs. 1.35 ± 0.32, p = 0.062), but the GLPSS was significantly decreased in Group I compared with Group II (Group I -10.3 ± 4.2% vs. Group II -13.1 ± 4.8%, p = 0.021). The WMSI of the infarct segments was significantly higher (2.13 ± 0.57 vs. 1.66 ± 0.57, p = 0.001) and the GLPSS of the infarct segments was significantly decreased in Group I compared with Group II (-3.7 ± 5.4 vs. -11.4 ± 4.8%, p < 0.001) (Fig. 2).
ROC curve analysis
The ROC curve analysis of the GLPSS and WMSI was done to identify optimal cut-off values and is shown in Fig. 3. The area under the curve was 0.695 for the GLPSS of all segments and 0.850 for the GLPSS of the infarcted segments. The optimal cut-off value to predict the occlusion of the IRA was -9.4% for the GLPSS of the infarcted segments, with a diagnostic sensitivity of 85.4% and specificity of 67.9%, and -13.1% for the GLPSS of all segments, with a diagnostic sensitivity of 73.2% and specificity of 64.3%. The area under the curve was 0.661 for the WMSI of all segments and 0.719 for the WMSI of the infarcted segments. The optimal cut-off value to predict the occlusion of the IRA was 1.68 for the WMSI of the infarcted segments, with a diagnostic sensitivity of 78.0% and specificity of 60.7%, and 1.34 for the WMSI of all segments, with a diagnostic sensitivity of 65.9% and specificity of 60.7%.
Impact of lesion location on GLPSS
GLPSS was -11.2 ± 4.4% in patients with infarction of the left anterior descending coronary artery territory (LAD), -13.5 ± 4.4% in patients with infarction of right coronary artery territory (RCA), and -13.8 ± 3.1% in patients with infarction of left circumflex coronary artery territory (LCx). GLPSS did not differ according to IRA location (p = 0.068 between LAD and RCA, p = 0.073 between LAD and LCx, and p = 0.829 between LCx and RCA). GLPSS was significantly decreased in patients with proximal IRA lesion locations compared with patients with mid to distal IRA lesion locations (-13.9 ± 4.1 vs. -10.8 ± 4.1%, p = 0.003).
DISCUSSION
The present study demonstrated that the GLPSS measured by AFI was a more sensitive predictor of coronary perfusion before PCI than was the WMSI in patients with AMI, and its diagnostic sensitivity improved when the GLPSS of the IRA was used. The present study also demonstrated that the patients with spontaneous reperfusion of the IRA before PCI had smaller infarcts than did the patients with coronary occlusion of the IRA, as evidenced by the more favorable values of the GLPSS and WMSI. Therefore, the result of this study suggested that the measurement of GLPSS by AFI can be a useful echocardiographic tool in risk stratification of patients with AMI before PCI.
In previous studies, spontaneous reperfusion of the IRA before PCI has been reported in up to 30% of patients with acute STEMI [5]. About 43% of patients in the present study also showed spontaneous coronary reperfusion of the IRA, defined as TIMI flow greater than grade 2 before PCI, and most of them had TIMI 3 flow. Considering the fact that the present study included both STEMI and NSTEMI, the incidence of spontaneous reperfusion in the present study may be similar to that of previous studies. Spontaneous reperfusion before mechanical reperfusion therapy and early restoration of normal coronary perfusion of the IRA are known to be significant predictors of prognosis following AMI [5-9,17,18]. Previous studies have demonstrated that AMI patients with spontaneous reperfusion of the IRA have smaller infarcts and more favorable prognoses [5-9]. Therefore, it is assumed that the early identification of the perfusion status of the IRA before coronary angiography may be useful in risk stratification and decision making with regard to treatment strategy in patients with AMI, especially in hospitals without on-site catheterization laboratories or in patients who have been under thrombolytic treatment.
A few studies have focused on non-invasive predictors of coronary reperfusion before coronary angiography in patients with AMI [19-22]. Clinical, laboratory, and electrocardiographic predictors were used in those studies, but these parameters are not reliable indices of coronary patency [19-22]. Although several echocardiographic parameters, such as WMSI and ejection fraction of the LV, have been introduced and used for risk stratification after AMI [23,24], few studies have been conducted to investigate the echocardiographic predictors of coronary reperfusion before PCI in patients with AMI. Iwakura et al. [14] reported that the analysis of myocardial integrated backscatter could detect spontaneous reperfusion non-invasively in the emergent stage of anterior AMI. Moir et al. [15] reported that the quantitative analysis of intravenous myocardial contrast echocardiography could predict impaired coronary flow in patients with acute STEMI. However, both analysis of myocardial integrated backscatter and contrast echocardiography are complex and not easy to use in general practice because they require specialized software or contrast agents.
Measurement of strain and strain rate by TDI has gained wide acceptance as a quantitative and accurate technique to evaluate regional and global myocardial function in various clinical settings, but has often been limited by a number of potential pitfalls, such as angle dependency and high frame-rate requirement [11,12]. Non-Doppler, 2D strain imaging based on a speckle tracking method has been introduced to overcome these limitations and has replaced the role of TDI in the measurement of strain values [10-13]. 2D strain imaging is a new technique that uses standard B-mode images for speckle tracking. Strain and strain-rate analysis have also been shown to be useful tools in evaluating regional ventricular function, infarct size, myocardial viability, and recovery after medical or mechanical reperfusion, as well as the subtle changes related to myocardial ischemia in patients with AMI [25-28]. However, the usefulness of strain analysis in the prediction of the perfusion status of IRA before PCI has been poorly evaluated. Therefore, we wanted to evaluate the usefulness of GLPSS analysis in the prediction of perfusion status of IRA before PCI using a newly introduced AFI technique. Only three apical views are needed for GLPSS analysis by AFI. By clicking three points in apical views, AFI noninvasively tracks and analyzes GLPSS based on a 2D speckle tracking method and displays the combined results of GLPSS of three planes in a single bull's eye summary. Therefore, AFI minimizes the variability potentially created in a manual tracing process and reduces the time required for the measurement of GLPSS. The results of the present study suggested that this simplified measurement of GLPSS by AFI may be a useful method in predicting the perfusion state of IRA. The optimal cut-off value of GLPSS of the infarcted segments to predict IRA occlusion was -9.4% by ROC curve analysis. The GLPSS of infarcted segments less than -9.4% significantly predicted the occlusion of IRA, with a diagnostic sensitivity of 85.4% and specificity of 67.9%, and PSS by AFI was a more sensitive predictor of IRA occlusion than was WMSI in the present study. Therefore, the results of the present study suggested that the simple and rapid measurement of GLPSS by AFI can be a useful tool in risk stratification and clinical decision making with respect to therapeutic strategies in patients with AMI.
Primary PCI and thrombolytic therapy within 12 hours are preferred and established therapeutic strategies in patients with acute STEMI [29]. Although early invasive strategies are recently preferred treatments in many cardiac catheterization laboratories, the ideal treatment strategy in patients with acute NSTEMI within 12 hours is still debated [30]. Because approximately 20% to 30% of the patients with acute NSTEMI had total occlusion of IRA [31], the NSTEMI patients with these clinical situations may benefit from early invasive treatment, similar to patients with acute STEMI. Therefore, the present study provides rationales for early invasive strategy in NSTEMI patients in these clinical settings. According to the results of this study, NSTEMI patients with PSS of the infarct segment less than -9.4% would have a higher chance of IRA occlusion and may benefit from early invasive PCI.
The present study has several potential limitations. First, although most echocardiographic examinations were done within 12 hours of AMI, all echocardiographic examinations were not done at the same time from the onset of AMI. The time interval from the onset of chest pain to PCI was not same in all patients, even though the present study included patients who underwent successful PCI within 12 hours from the onset of AMI. Therefore, the strain values might be affected by these time differences among echocardiographic examinations or PCI procedures because the difference in the time interval from the onset of AMI to echocardiographic examination or PCI may represent the difference of the duration of IRA occlusion. Because most echocardiographic examinations were done before PCI, and the remainder were also done within 1 hour after PCI, the effect of PCI on strain values might be minimized in the present study. Second, the impacts of IRA location should be considered in the interpretation of the results of the present study, because it is known that LAD territory infarction may have a larger infarct size than RCA or LCx territory infarction. GLPSS did not differ according to the location of IRA in present study, although LAD territory infarct had a tendency toward lower GLPSS compared with LCx or RCA territory infarcts. Furthermore, the distributions of IRA did not differ between the groups. Therefore, the impacts of IRA location on strain values would be minimized. Third, GLPSS may differ according to the lesion location within the same IRA. Actually, GLPSS was significantly decreased in patients with proximal lesions of IRA compared with that in patients with mid to distal lesions of IRA in the present study. However, the lesion locations did not differ in the present study. Therefore, the impact of lesion location on strain values would be minimized. Fourth, the TIMI flow grade 0 or 1 was defined as the occlusion of the IRA, and TIMI flow 2 or 3 as the patent IRA in the present study; however, TIMI flow grade 2 is also not a complete perfusion status of the IRA. Therefore, it should be noted in the interpretation of the present study that a patent IRA does not always signify complete reperfusion of the IRA. Fifth, recovery of the regional wall-motion abnormality evaluated by WMSI or strain following AMI may be delayed, even after successful restoration of IRA perfusion by PCI (so-called myocardial stunning). Therefore, the impact of myocardial stunning should be considered in the interpretation of the results of the present study.
In conclusion, the present study demonstrated that GLPSS measured by AFI is a more sensitive predictor of IRA occlusion than is WMS or WMSI before PCI. Therefore, the routine measurement of GLPSS by AFI can be a very useful tool in risk stratification or clinical decision making with regard to patients with AMI, especially in hospitals without on-site catheterization laboratories, and in the evaluation of reperfusion of patients under thrombolytic treatment.
Notes
No potential conflict of interest relevant to this article was reported.