Serum C-Reactive Protein Levels in Normal-Weight Polycystic Ovary Syndrome
Article information
Abstract
Background/Aims
Serum levels of highly sensitive C-reactive protein (hsCRP), a vascular inflammatory marker, may predict the development of cardiovascular disease (CVD) and type 2 diabetes. Women with polycystic ovary syndrome (PCOS) are at greater risk for type 2 diabetes and CVD. The aim of this study was to compare hsCRP levels between normal weight women with PCOS and controls with a normal menstrual cycle and to determine the factors associated with serum hsCRP levels.
Methods
Thirty-nine lean PCOS patients and 24 healthy, regular cycling women were enrolled in this study. We performed anthropometric measurements, fat computed tomography (CT), and blood sampling to determine blood chemistry and levels of hsCRP, gonadotropins, testosterone, and sex-hormone binding globulin. We also conducted 75-g oral glucose-tolerance test and euglycemic hyperinsulinemic clamp to assess insulin sensitivity.
Results
Serum hsCRP concentrations were higher in women with PCOS than in women with regular mensturation. However, this difference was no longer significant after adjusting for body mass index (BMI). hsCRP levels were correlated with waist circumference (r=0.46, p<0.01), BMI (r=0.46, p<0.01), visceral fat area (r=0.45, p<0.01), and systolic (r=0.42, p<0.05) and diastolic blood pressure (r=0.39, p<0.05). hsCRP also tended to be negatively associated with insulin-mediated glucose uptake (IMGU) (r=-0.31, p=0.07). A multiple regression analysis revealed that BMI (β=0.29, p<0.05), systolic blood pressure (β=0.39, p<0.01), and IMGU (β=-0.31, p<0.05) predicted serum hsCRP levels in women with PCOS.
Conclusions
PCOS by itself does not seem to be associated with increased hsCRP levels, whereas known CVD risk factors affect serum hsCRP levels in PCOS.
INTRODUCTION
Polycystic ovary syndrome (PCOS) is a common endocrine disorders that affects 5-10% of reproductive-age women [1,2]. Insulin resistance is considered the most important pathophysiological factor in PCOS [3-5]. The cellular and molecular mechanisms of insulin resistance in PCOS have not yet been elucidated, but they are considered to be distinct from those of other diseases associated with insulin resistance [6]. Over half of women with PCOS are obese [7] and show insulin resistance [8,9]. Therefore, they have an increased prevalence of hypertension, diabetes, and cardiovascular disease (CVD) [10,11]. Serum highly sensitive C-reactive protein (hsCRP), a marker of chronic subclinical inflammation, is a well-known predictor for the development of type 2 diabetes and CVD [12]. It has been reported that serum hsCRP levels increase in metabolic disorders such as obesity, hypertension, dyslipidemia, and atherosclerosis [12]. A few studies have examined CRP levels in women with PCOS; however, the results are not consistent. Some studies report that CRP levels are increased in women with PCOS [13-15], supporting the hypothesis that the risk of diabetes and CVD is increased by chronic inflammation in PCOS. In contrast, Mohlig et al. [15] reported that serum CRP levels are associated with obesity rather than with the presence of PCOS per se. Thus, whether serum CRP levels are increased in women with PCOS and which variables predict serum CRP levels remain unclear.
The purpose of this study was to compare hsCRP levels between lean women with PCOS and women with normal menstrual cycling, and to investigate the relationship between serum hsCRP and other CVD risk factors in women with PCOS.
METHODS
Subjects
Thirty-nine lean (body mass index [BMI] <23 kg/m2) women with PCOS and 24 regular-cycling control women were enrolled in this study. The degree of obesity was classified according to the Asia-Pacific perspective, 2000 [16]. None of the control women had a family history of diabetes or PCOS. The diagnosis of PCOS was made according to the National Institute of Child Health and Human Development (NICHD) criteria [17], i.e., hyperandrogenism with amenorrhea or oligomenorrhea (less than nine menstrual cycles per year) and the exclusion of specific disorders such as adult-onset congenital adrenal hyperplasia, hyperprolactinemia, and androgen-secreting neoplasia. Hyperandrogenemia was defined as serum free testosterone levels greater that the 90th percentile (>52.2 ng/dL) in women with regular menstrual cycles and no hirsutism.
Anthropometric measurements
Height and weight were measured with subjects wearing lightweight clothing and without shoes. BMI was calculated as weight (kg)/height (m2). Waist circumference was measured on bare skin during mid-respiration at the narrowest indentation between the 10th rib and the iliac crest. Blood pressure was measured twice using sphyngomanometry following a 10 minute-rest, and mean values are presented. For the measurement of visceral fat area (VFA), computed tomography was performed at the umbilicus level, and fat was defined as pixels within a window of -144 to -44 Houndsfield units.
Baseline sampling and oral glucose tolerance test
Venous blood sampling was performed to assess baseline measurements of hsCRP, glucose, lipid, insulin, testosterone, sex hormone binding globulin (SHBG), luteinizing hormone (LH), and follicle stimulating hormone (FSH) following an overnight fast during the early follicular phase. Blood samples for oral glucose tolerance tests were obtained at 30 minutes intervals for 2 hours following ingestion of a standard dose of glucose (75 g) to measure glucose and insulin levels.
Glucose clamp technique
Insulin sensitivity was evaluated using a modified version of hyperinsulinemic euglycemic clamp techniques described by DeFronzo et al. [18]. A quantitative estimate of insulin sensitivity, glucose disposal rate, and insulin-mediated glucose uptake (IMGU) was determined by recording the mean glucose infusion rate (mg/kg/min) for the last 15 minutes of a 2-hour hyperinsulinemic euglycemic clamp.
Assay methods
Analysis of hsCRP was performed using immunonephelometric methods and a BN-II analyzer (Dade Behring, Deerfield, Germany). The inter- and intra-assay coefficients of variation were 4.9 and 6.8%, respectively.
Plasma glucose levels were measured using the glucose oxidase method (Beckman Model Glucos Analyzer 2; Beckman, Los Angeles, CA, USA). Plasma insulin levels were determined using a radioimmunoassay (RIA) kit (Diagnostic Products Corporation [DPC], Los Angeles, CA, USA). Total cholesterol (TC), HDL cholesterol (HDL-C), and triglycerides (TG) were measured via enzymatic methods using a Hitachi 7150 autoanalyzer (Hitachi, Tokyo, Japan). LDL cholesterol was calculated as TC (mg/dL) - HDL-C (mg/dL) - TG (mg/dL)/5. Serum testosterone, SHBG, LH, and FSH were measured using RIA kits from DPC. Free testosterone levels were calculated using a formula available on the website of the International Society for the Study of Aging Male (http://www.issam.ch/freetestos.htm) based on total testosterone, SHBG, and albumin values from each subject [19].
Statistical analysis
Data analysis was performed using SPSS version 12.0 (SPSS Inc., Chicago, IL, USA). Data showing a skewed distribution were logarithmically transformed prior to statistical analysis. Data are expressed as the mean± standard deviation, unless otherwise stated. A p value of <0.05 was considered statistically significant.
An unpaired t-test was used for comparison of continuous variables between PCOS and control groups. A multivariate general linear model was applied for comparisons between two groups after adjusting for specific variables. Linear correlations were examined using Pearson's correlation. A multiple regression analysis was performed to determine which variables predict serum hsCRP levels.
RESULTS
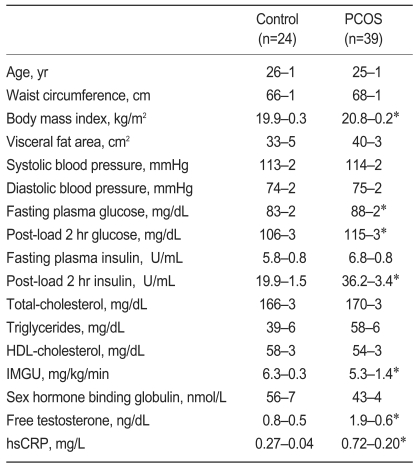
Subject characteristics are summarized in Table 1. Age, waist circumference, VFA, and systolic and diastolic blood pressures were not significantly different between PCOS and control groups. The PCOS group had a higher BMI (20.8±0.2 vs. 19.9±0.3 kg/m2, p<0.05); however, BMI in all subjects was less than 23 kg/m2. In women with PCOS, fasting plasma glucose (88±2 vs. 83±2 mg/dL, p<0.05), 2 hour post-load plasma glucose (115±3 vs. 106±3 mg/dL, p<0.05), 2 hour post-load plasma insulin (36.2±3.4 vs. 19.9±1.5 µU/mL, p<0.05), and free testosterone levels (1.9±0.6 vs. 2.8±0.4 ng/dL, p<0.05) were higher greater compared with the control group, and IMGU (5.3±1.4 vs. 6.3±0.3 mg/kg/min, p<0.05) was lower. hsCRP concentrations in the PCOS group were higher compared with the control group (0.72±0.20 vs. 0.27±0.04 mg/L, p<0.05); however, this difference was not statistically significant after adjusting for BMI (data not shown).
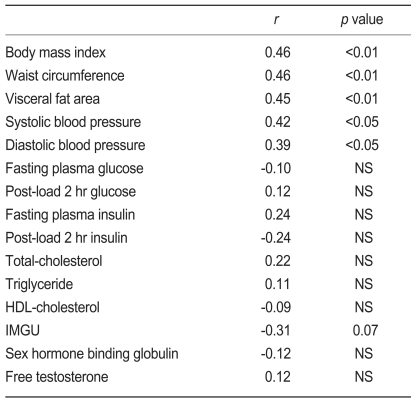
hsCRP levels were positively correlated with waist circumference (r=0.46, p<0.01), BMI (r=0.46, p<0.01), VFA (r=0.45, p<0.01), systolic (r=0.42, p<0.05) and diastolic and blood pressure (r=0.39, p<0.05), and tended to be negatively correlated with IMGU (r=-0.31, p=0.07) in women with PCOS (Table 2).
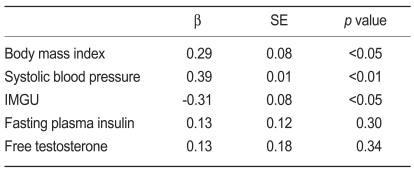
A multiple regression analysis showed that BMI (β=0.29, p<0.05), systolic blood pressure (β=0.39, p<0.01), and IMGU (β=-0.31, p<0.05) predicted serum hsCRP levels in women with PCOS (Table 3).
DISCUSSION
The purpose of this study was to compare the levels of hsCRP in lean women with and without PCOS, and determine whether hsCRP is associated with other CVD risk factors. The present analysis suggests that PCOS is not associated with increased CRP levels. In contrast, documented CVD risk factors appear to predict serum CRP levels in women with PCOS. In this study, we recruited only normal-weight women with PCOS to minimize the effects of obesity on hsCRP levels.
PCOS is characterized by chronic anovulation, hyperinsulinemia, hyperandrogenism, and infertility, and it is one of the most frequently observed endocrine disorders in reproductive-age women [8]. PCOS shares many characteristics of metabolic syndrome, including abdominal obesity, insulin resistance, hypertension, dyslipidemia, and atherosclerosis [20-22]. Wild et al. [23] reported that PCOS women have a high prevalence of nonfatal cerebrovascular disease and cardiovascular risk factors, including diabetes, hypertension, hypercholesterolemia, hypertriglyceridemia, and increased waist-to-hip ratio compared with control women. Furthermore, Elting et al. [24] recently reported that in women aged 45-54 years with PCOS, the prevalence of diabetes mellitus is four times higher and the prevalence of hypertension is 2.5 times higher as compared with control women in the corresponding age group within a Dutch population. In contrast, one epidemiologic study did not find any differences in either all-cause or cardiovascular mortality in women who had been classified as having PCOS in the past [25].
It has been reported that surrogate markers of cardiovascular risk such as tumor necrosis factor-α, homocystein, and plasminogen activator inhibitor-1 are increased in women with PCOS [26-28]. Several large-scale prospective studies have shown that CRP is a strong independent predictor of future CVD and/or stroke [12,29]; however, only a few studies have determined CRP levels in women with PCOS. Kelly et al. [13] reported that women with PCOS have significantly increased CRP concentrations compared with women who demonstrate normal menstrual cycles and normal androgen levels. In contrast, Mohlig et al. [15] did not observe these same findings in women with PCOS.
In the present study, hsCRP concentrations in the PCOS group were higher than in the control group; however, this effect disappeared after adjusting for BMI. In women with PCOS, hsCRP levels were correlated with CVD risk factors such as waist circumference, BMI, VFA, systolic and diastolic blood pressures, and IMGU. Multiple regression analysis showed that BMI, systolic blood pressure, and IMGU predicted increased hsCRP levels in PCOS women.
As previously reported, it appears that obesity is a major factor associated with elevated CRP in individuals with metabolic syndrome [30]. Moreover, visceral fat correlates with CRP concentrations independently of total adiposity [31].
Hyperandrogenemia in PCOS can alter body fat distribution and result in central obesity, which may affect insulin sensitivity in women. We did not observe any association between hsCRP and free testosterone levels after adjusting for BMI, blood pressure, and IMGU. Therefore, it seems unlikely that hyperandrogenemia is linked to chronic inflammation in women with PCOS. These results are consistent with previous studies that showed no correlation between testosterone and CRP levels after adjusting for VFA [13,15]. Therefore, further studies are required to identify potential mechanisms underlying the relationship between CRP and testosterone concentrations in women with PCOS.
In this study, CRP levels in women with PCOS were lower than in previous reports [13-15]. Boulman et al. [14] reported that PCOS women with CRP concentrations greater than 3 mg/L had a higher prevalence of moderate cardiovascular risk compared with BMI-matched controls. hsCRP levels in the PCOS women in our study were below this level (0.72±0.20 mg/L). Albert et al. [32] reported that the distribution of CRP levels varied significantly by ethnic group. In this study, Asian women showed lower CRP levels as compared with Caucasian and Hispanic women. This may partially explain the relatively low CRP levels in our subjects. Moreover, the women with PCOS who participated in our study were quite young (17-35 years) and may have a relatively smaller risk of CVD compared with other studies. Collectively, these factors appear to contribute to the low CRP levels observed in this study. Therefore, age, BMI and gender should be considered when interpreting CRP data [33,34].
In conclusion, our study showed that PCOS is not associated with increased CRP levels, but rather, documented risk factors for CVD such as BMI, blood pressure, and insulin sensitivity predict CRP levels in PCOS.
Acknowledgements
This study is partly supported by grant from Ewha Womans Univsersity alumni foundation.