Thalidomide for Treating Metastatic Hepatocellular Carcinoma: A Pilot Study
Article information
Abstract
Background
Thalidomide has been reported to have antitumor activity for treating metastatic hepatocellular carcinoma (HCC). We evaluated the safety and efficacy of using thalidomide for treating selected patients with unresectable or metastatic HCC, and their disease was refractory to systemic chemotherapy.
Methods
Eight patients with measurable and metastatic HCC that had progressed with prior systemic chemotherapy and who desired further active therapy were enrolled in this study. Thalidomide was given orally at bedtime and it was started at 200 mg/day with no further dose escalation. The response was measured at 2-month intervals.
Results
The median age was 44 years (range: 34-52 years) and all the patients had received doxorubicin-based systemic chemotherapy prior to their enrollment. Each patient received thalidomide for a median of 152 days (range: 5-422 days). One partial response was observed (12.5%, 95% CI; 0-42%) along with 4 cases of stable diseases. The most commonly encountered toxicity was somnolence; grade 3 somnolence was noted for one patient, which led to treatment discontinuation. Skin rash was observed in one responding patient.
Conclusions
The results indicate that thalidomide may feasibly offer disease stabilization to metastatic HCC patients. Further dose escalation of thalidomide, or its combination with other chemotherapeutic agents, may be of interest and this should be investigated for treating patients with metastatic HCC.
INTRODUCTION
Hepatocellular carcinoma (HCC) accounts for 12% of all malignancies in Korea and it is one of the leading causes of cancer death1). Metastatic HCC is associated with a very poor prognosis as it often develops among cirrhotic patients. It is estimated that approximately 80% of all patients with HCC have underlying cirrhosis2). In some patients, cirrhosis associated with portal hypertension and thrombocytopenia makes administering systemic chemotherapy for HCC extremely difficult and this contributes to the poor prognosis associated with HCC. Furthermore, the role of systemic chemotherapy for metastatic HCC has not yet been clarified3, 4). With few exceptions, it is generally though that systemic chemotherapy is relatively ineffective in HCC5). HCC is resistant to chemotherapy because of the diseases high mutational load and its myriad of drug resistance mechanisms6, 7). This is in addition to the underlying liver dysfunction that imposes requirements for lower chemotherapy doses to mitigate toxicity.
Thalidomide is an immunomodulatory agent that blocks angiogenesis, inhibits cytokines (tumor necrosis factor-á, basic fibroblast growth factor and vascular endothelial growth factor) and modifies the expression of cell adhesion molecules8, 9). Based on this activity, thalidomide has successfully been applied for the treatment of various malignancies including multiple myeloma10). Several recent studies have indicated the activity of thalidomide in HCC11-14) at the cost of considerable dose-dependent toxicity, and especially somnolence, constipation, lethargy and thromboembolism; neurotoxicity has been reported with prolonged therapy.
We hypothesized that thalidomide may be a promising agent for inhibiting HCC tumor growth. Thus, we conducted a pilot study to evaluate the activity of this agent in HCC patients.
MATERIALS AND METHODS
The patients were required to have histologically confirmed and radiologically measurable metastatic HCC that was progressive after previous anthracycline-based chemotherapy, but they desired further active therapy. Their age was less than 75 years. They had an Eastern Cooperative Oncology Group (ECOG) performance status = 2, an absolute neutrophil count =1,000/mm3, a platelet count =40,000/mm3, a serum creatinine level =2 mg/dL and a Child-Pugh class A or B15). Women who were pregnant or breast-feeding were ineligible for the treatment. Patients who had evidence of peripheral neuropathy, prior thalidomide exposure or any active infection that was not effectively controlled with antibiotics were defined as ineligible. The patients were informed that treatment with thalidomide constituted off-label use of the agent. All the patients received information regarding the potential benefits and toxicities associated with thalidomide, and all of them gave us a written informed consent according to the guideline provided by the Gil Medical Center institutional Review Board.
Treatment plan
Thalidomide was orally administered on an outpatient basis at bedtime; it was started at 200 mg/day with no further dose escalation. A thorough physical examination, complete blood counts, blood chemistry and the serum alpha-fetoprotein (AFP) level were obtained at baseline and at one-month intervals. Toxicity was classified according to the National Cancer Institute Common Toxicity Criteria version 2.0. Thalidomide was interrupted in the event that unacceptable somnolence was documented. If grade =2 neuropathy was observed, then thalidomide was discontinued. Otherwise, treatment was continued until disease progression was documented. In cases of active viral replication, antiviral treatment (i.e., lamivudine) was permitted.
Evaluation and statistical analysis
The antitumor response was assessed at a two-month intervals and this was later reviewed by an independent investigator at the time of analyses. The following criteria were used: [1] a complete response (CR) was defined as the absence of clinically detectable tumor and normalization of the serum AFP levels for at least 4 weeks; [2] a partial response (PR) was defined as a = 50% reduction in the sums of the products of the bidimensional tumor measurements and/or a reduction = 50% of the serum AFP levels; [3] stable disease (SD) was defined as an increase of < 25% or a decrease of < 50% in the sums of the products of the bidimensional tumor measurements and also for the serum AFP levels. Progressive disease (PD) was defined as an increase of = 25% in the sums of the products of the bidimensional tumor measurements and/or an increase of = 25% in the serum AFP levels.
Overall survival (OS) was defined as the time that elapsed from the starting date of thalidomide treatment to the date of death or the last follow-up, and this was assessed by the KaplanMeier method. All statistical analyses were performed using the SPSS for Windows 11.5 statistical package (SPSS Inc., Chicago, IL).
RESULTS
Patients
Between August 2003 and March 2005, eight patients were treated with thalidomide. The patients characteristics are summarized in Table 1. The median age was 44 years (range: 34 to 52 years), and all of them had received doxorubicin-based systemic chemotherapy for metastatic HCC. All the patients had at least one metastatic lesion with intra-abdominal lymph nodes, lung and bone metastases present in 5, 3 and 2 patients, respectively. Four patients had portal vein thrombosis and 5 were HBsAg positive.
Efficacy
One patient was not evaluable for response. The patient, a 41-year-old male, displayed considerable disorientation and confusion after 5 days of treatment and he refused further treatment. Among the remaining 7 evaluable patients, PD was observed in two patients (No. 4 and No. 7; Table 1); PR was observed in only one patient (No. 1). He had been previously treated with a doxorubicin plus cisplatin regimen (AP) before participating to this trial. After one cycle of AP, thrombocytopenia and early progression of disease was observed. Simultaneously, spinal cord compression occurred and this was treated by radiation and high dose steroid therapy for about 20 days. He presented with metastases in bone and the intra-abdominal and supraclavicular lymph nodes; his Child-Pugh class was B. He received a total of 307 days of thalidomide treatment and a significant reduction (58,682 to 1,638 ng/mL) was noted in the serum AFP level (Figure 1); there was also a remarkable decrease of the multiple abdominal lymph nodes in their extents and sites after 58 days of treatment (Figure 2). The PR was maintained for 8 months.

Abdominopelvic CT scans of patient no. 1, which shows multiple abdominal lymph node enlargements at diagnosis (upper) and a remarkable decrease of multiple abdominal lymph nodes in the extent and sites after 58 days of treatment (below).
SD was observed in four patients. The first patient with SD (No. 3) had presented with metastatic lymph nodes and PD after 2 cycles of systemic chemotherapy (doxorubicin, cisplatin and capecitabine). The second patient (No. 5) had also suffered from PD after 5 cycles of the above mentioned chemotherapy, and this patient presented with bone and lymph node metastases. They both received a total of 103 and 92 days of thalidomide treatment, respectively, resulting in ongoing SD at all metastatic sites. The third patient (No. 6) had presented with asymptomatic lung metastasis and PD after 9 cycles of doxorubicin plus cisplatin chemotherapy. He refused further treatment after 422 days for financial reasons, but up to now, he still remains in SD. The fourth patient with SD (No. 8) had been treated with combination chemotherapy of doxorubicin, cisplatin and capecitabine, and he had responded for up to 6 months. He was enrolled in the present study with showing evidence of PD in the paraaortic lymph nodes. His performance status improved considerably under treatment. The toxicity was moderate with grade 2 fatigue and somnolence. After 150 days of treatment, he died of hypovolemic shock that occurred with esophageal variceal bleeding, which was a complication of his underlying cirrhosis.
The median OS for all patients was 395 days (range: 46-422 days). Currently, 3 patients are still alive (2 with SD and one with PD).
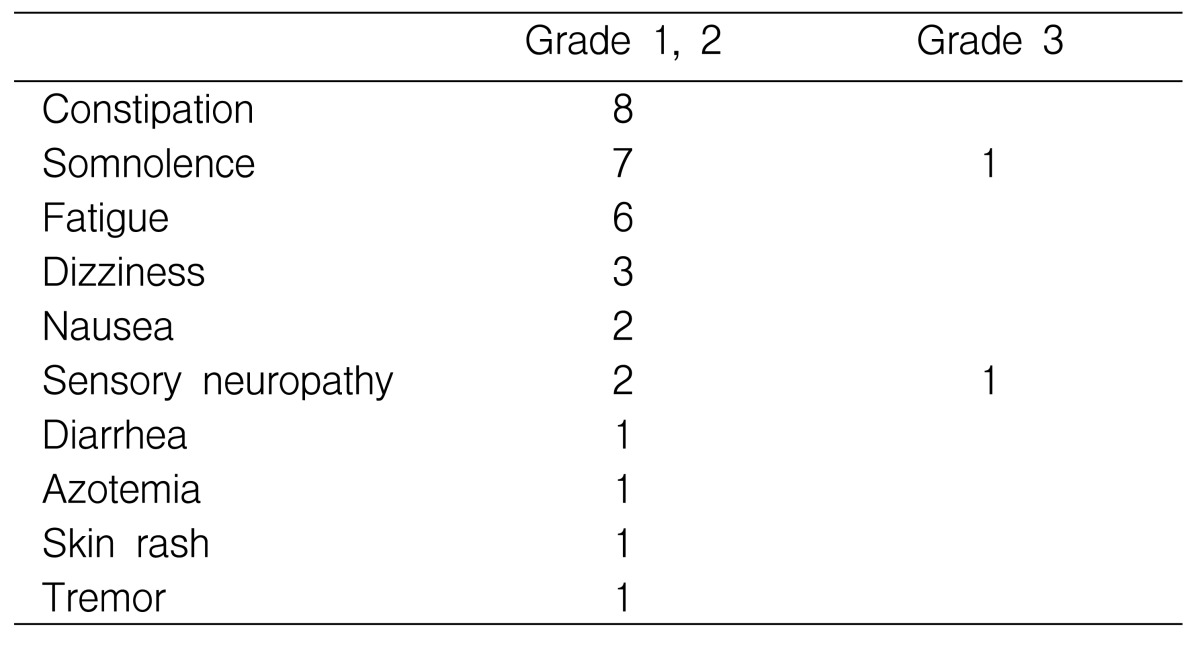
Safety
All the patients experienced a depressed level of consciousness (somnolence and/or sedation) and constipation (Table 2). The somnolence and fatigue were predominantly moderate, and fatigue was most prominent at the beginning of treatment. Skin rash was observed in the one responding patient. All the observed toxic effects of thalidomide disappeared when the medication was discontinued, except for one case of skin rash that persisted until the patients death. None of the patient suffered clinically significant hematologic toxicity or venous thromboembolism. The time on therapy ranged from 5 to 422 days (median: 152 days).
DISCUSSION
Systemic therapy is the treatment of last resort for patients with metastatic HCC. A recent review of multiple studies detected no reproducible benefit associated with systemic therapy for the patients suffering with metastatic HCC5). More recently, targeted therapeutics are being aggressively looked at for treating HCC. Several potential targets along the angiogenesis and the signal transduction pathways are being evaluated in HCC. These include epidermal growth factor receptor, hepatocyte growth factor and its receptor c-met, and vascular endothelial growth factor16). Thalidomide, which has been known to inhibit growth factor-induced neovascularization, is currently being tested in multiple phase II clinical studies17). This drug is perceived as an oral agent that is relatively nontoxic and it possesses some clinical activity; thalidomide is a convenient alternative to treatment with using cytotoxic agents. However, despite the appealing rationale for the use of thalidomide as a treatment of hypervascular tumors such as HCC, two recent studies have demonstrated that only a minority of cases show an objective tumor response to this drug18, 19). Moreover, escalation to higher doses does not appear beneficial for tumors that are refractory to low doses (200~300 mg/day) and this dose increase markedly increases the drug toxicity20).
In this pilot study, thalidomide treatment was well tolerated by the patients with metastatic HCC and who had failed with previous doxorubicin-based systemic chemotherapy. With oral thalidomide 200 mg/day, we achieved a PR of 12.5% (95% CI; 0~45%) and a median OS of 395 days. This observation should be interpreted with caution because it represents only a small group of patients with metastatic HCC in this pilot study and the majority of patients had a good performance status and a preserved hepatic function. However, we might have underestimated the responses by selecting only the patients who had been previously treated with systemic chemotherapy. The response rates for systemic therapy that is subsequent to a first-line regimen in patients with metastatic disease are lower. Furthermore, it is unlikely that second-line therapy in such patients will result in substantial prolongation of survival, and there is the potential for toxicity from thalidomide treatment. The initial treatment plan, in which dose escalation was not allowed, forced us to reduce the dose intensity of thalidomide so that the achieved daily dose was less than those utilized by other authors. The toxic effects of thalidomide were principally neurological (Table 2). Most adverse events in this pilot study could be managed by standard supportive care and/or treatment interruption. The incidence and severity of sensory neuropathy compared well with the other previous phase II studies18, 19). For cirrhotic HCC patients, neuropathy can be related, in part, to subclinical dysfunctions of the central and peripheral nervous system, which is frequently seen in cirrhosis21). In conclusion, thalidomide may feasibly offer disease stabilization for HCC patients. It is possible that longer patient survival might be expected with an escalated dosage, or when thalidomide is combined with other chemotherapy agents. Further evaluation of the efficacy of thalidomide for treating HCC is needed both as a single agent in the less pretreated patients, as well as in combination with other chemotherapeutic agents.