Immunomodulation Therapy for Severe Aplastic Anemia:
—ALG Versus ALG Plus Cyclosporin A—
Article information
Abstract
Immunosuppressive treatment of aplastic anemia has been increasingly used as an alternative treatment to bone marrow transplantation. In this study, the additive effect of Cyclosporin A (CSA) (5mg/kg/day, at least 3 months) for maintenance of immunosuppression after antilymphocyte globulin (ALG) therapy (40mg/kg/day for 4 days) was compared to the previous ALG alone treatment (15mg/kg/day for 10 days). A high dose of methylprednisolone (20mg/kg/day for 5 days and 10mg/kg/day for 5 days) to the ALG group and a low dose of methylprednisolone (2mg/kg/day for 5 days) to the ALG plus CSA group were administered simultaneously.
The results were as follows:
Sixteen (69.6%) out of twenty-three patients treated with ALG plus Cyclosporin A showed higher responses (CR: 48%, PR: 22%). On the contrary, nine out of nineteen in the ALG group showed lower responses (CR: 21%, PR: 26%).
Our data showed a tendency that male patients in age ranging from sixteen to thirty years showed an excellent response to ALG therapy (12/23: 52.2%).
The ALG plus CSA group revealed a faster response compared to the ALG alone group (15/16 within 6 months).
We speculate that ALG plus CSA therapy might be the treatment of choice for patients with a moderate degree of aplastic anemia.
Adding CSA to ALG increased the chance of infection, such as those with URI-like symptoms, but it did not affect the mortality rate.
Our data suggest that the ALG plus CSA regimen may be a more useful therapeutic modality for patients with severe aplastic anemia who cannot be candidates for bone marrow transplantation and a randomized multicenter study is needed for confirmation of our preliminary study.
INTRODUCTION
Aplastic anemia is characterized by peripheral pancytopenia due to bone marrow failure. In severe cases, more than 50 % of patients succumb to death within six months. The annual incidence of this disease is reported to be 10 – 30 per million in the Western hemisphere,1) but it seems to be 3 to 5 times more prevalent among the Oriental population.2)
Although the exact pathogenesis of aplastic anemia is still debated, there have been numerous reports suggesting immune mediated marrow failure. Engraft failures were clinically observed in approximately half of the cases of syngeneic bone marrow transplants in the absence of immunosuppressive conditioning,3,4) and autologous marrow recovery occurred in some patients who lacked a matched sibling.5) Abnormal T lymphocyte function has been implicated in the pathogenesis of the disease. Increased numbers of circulating Tsuppressor / cytotoxic cells capable of inhibiting growth of hematopoietic progenitors have been found in patients with aplastic anemia.6,7) Moreover, high gamma-interferon levels have been detected in the plasma and in the supernatant of T-cells of aplastic patients, suggesting that this lymphokine might be the mediator responsible for hematopoietic suppression.8) Antilymphocyte globulin (ALG) has an establihsed role in the treatment of severe aplastic anemia, especially for patients who do not have an HLA-identical sibling donor.9) Clinical response rates vary between 40% and 80%.10,11) In an attempt to increase the response rate to ALG therapy, we employed cyclosporin A (CSA) to maintain the immunosuppression that had been induced by ALG therapy. In this study, we compared the results between ALG alone and ALG plus CSA therapy.
MATERIALS AND METHODS
1. Materials
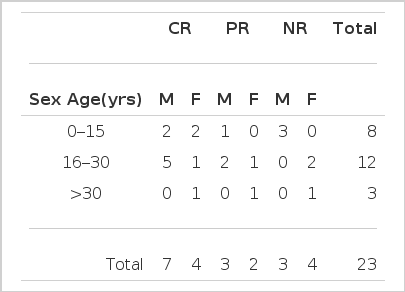
Among 86 patients with aplastic anemia who had been admitted to St. Mary’s Hospital from March 1984 to December 1986, 42 patients who could be followed for more than six months were enrolled in the ALG study. Nineteen patients were treated with ALG alone, and twenty-three were treated with ALG plus cyclosporin A. Between the two groups, there was no difference in distributions of age and sex (Table 3). The patients with Fanconi’s anemia, acquired aplasia from chemotherapy or radiotherapy, glycogen storage disease and hypersensitivity to the ALG test were excluded from our ALG study.
2. Diagnosis of Severe Aplastic Anemia and Definition of Response to ALG Therapy
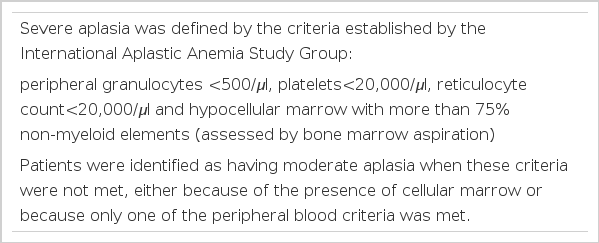
Severe aplasia was defined by the criteria established by the International Aplastic Anemia Study Group, ie; peripheral granulocytes less than 500 per microliter, platelets less than 20,000 per microliter, reticulocytes less than 20,000 per microliter, with bone marrow hypocellularity. The patients were classified as having moderate aplasia when these criteria were not met, either because of the presence of cellular marrow or because only one of the peripheral blood criteria was met (Table 1).
Clinical responses were divided into three groups. Complete response (CR) was defined when the granulocyte count was more than 1,000 and the platelets were more than 100 thousand per microliter, with return of a normal hematocrit. Partial response (PR) was defined when the granulocyte count was more than 500 per microliter without evidence of infection, but transfusions were still required. Non-response (NR) was defined when the patient remained severely aplastic at three months or died (Table 2).
3. Immunomodulation Protocol
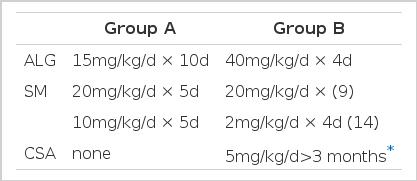
Patients were divided into two groups depending on the inclusion or not of cyclosporin A. For the group A patients, we administered 15mg/kg/d of ALG for 10 consecutive days simultaneously with 20mg/kg/d of Solu-Medrol for 5 days followed by 10mg/kg/d for five consecutive days. The group B patients received 40mg/kg/d of ALG for four days with 20mg/kg/d of Solu-Medrol for four days. Among the 23 in group B, 14 were treated with 2mg/kg/d, rather than 20mg/kg/d, of Solu-Medrol while receiving ALG therapy. We added 5mg/kg of oral cyclosporin A for at least three months to the group B patients starting from day 9 after ALG infusion to maintain the immunosuppression. Most of our patients were maintained with 2.5mg/kg/d of cyclosporin after six months (Table 4).
RESULTS
1. Response to the ALG Therapy
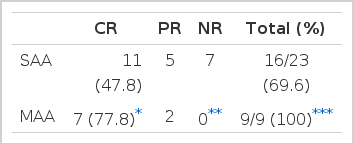
Sixteen (69.6%) out of the twenty-three patients treated with ALG plus cyclosporin A showed responses (CR: 48%, PR: 22%). On the contrary, nine out of the nineteen (47.4%) treated with ALG alone showed responses (CR: 21 %, PR: 26%). The difference in response rate was statistically significant (p<0.05) (Table 5).
2. Factors Influencing the Response Rate
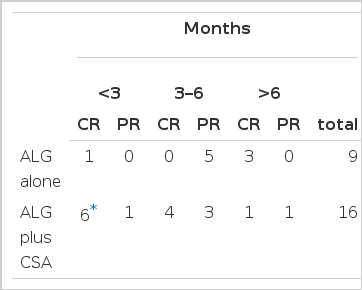
Six out of sixteen patients treated with ALG plus cyclosporin A showed faster responses within 3 months after initiation of therapy (p<0.01). Most of the patients in group B responded in six months. By contrast, there was a tendency of delayed response from three months to a year in the ALG alone group (Table 6).
-
Age and Sex
Our data suggested a tendency that male patients in age from sixteen to thirty showed excellent response to ALG plus cyclosporin therapy (12/23; 52.2%), but there was no statistical significance (p>0.05) (Table 7).
-
Dose of Steroid
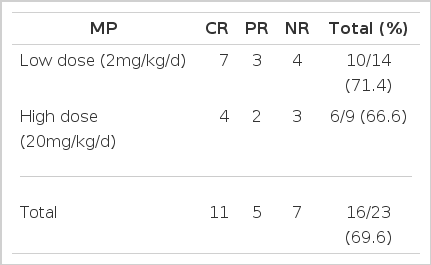
During our initial clinical trial using a high dose of solu-Medrol (20mg/kg/d) which was simultaneously infused with ALG therapy, the fluid retention worsened. Thus, we thereafter reduced the steroid dose to the level which would minimize the side effect of ALG infusion. Among twenty-three patients treated with ALG plus cyclosporin, fourteen patients were treated with low-dose steroid. The response rate was not significantly different in the two groups, and the low-dose steroid was revealed to be as effective as the high-dose in terms of providing the chance of response and protecting from ALG side effects (Table 8).
-
Severity of Aplastic Anemia
We applied the same protocol to the 9 patients with a moderate degree of aplastic anemia and we had a chance to compare the response rate between the two groups. To our surprise, all nine patients responded to ALG plus cyclosporin therapy, moreover in 7 a complete response were achieved in three months (Table 9).
-
Side Effects
Most of the patients developed fever and thrombocytopenia. One-third of the patients experienced transient hyperglycemia, hypertension, hypotension, and respiratory difficulty. The addition of cyclosporin increased the chance of fungal and viral infection and two cases of tuberculous pleurisy and four of pneumonia developed, but there was no clear evidence of increasing mortality (Table 10).
Response rate between Low Dose and High Dose Methylprednisolone (Solu-Medrol) in the ALG plus CSA Group (23 patients).
DISCUSSION
Although we have known that the CFU-C, BFU-E, and CFU-E were reduced in number in aplastic anemia, the exact mechanism by which the hematopoietic progenitors were decreased and why they lost their regenerating capability have never been understood clearly. The immune mechanism of hematopoietic suppression has been based upon the observation that syngeneic bone marrow transplants required immunosuppressive conditioning in 50% of severe aplastic anemia cases for successful engraftment4) and that immunosuppressive therapy could induce autologous marrow recovery in some patients who lacked the HLA-matched donors.5) In vitro studies suggested that the patient’s T cells suppress the hemopoietic progenitor colonies12,13,14) and removal of the T cells from the cultures restored the growth of the CFU-GM and CFU-E. Antilymphocyte globulin (ALG) or antithymocyte globulin (ATG), cyclophosphamide, methylprednisolone and cyclosporin have been administered in a single or combined manner with varying results.9,12,15–20) The authors have already experienced the ALG effect in 24 cases of aplastic patients.21)
In order to increase the response rate, we introduced cyclosporin A to maintain the immunosuppression which was induced by ALG therapy. The exact mechanism of immunosuppressive agents in aplastic anemia still remains to be clarified. Glucocorticoids are lymphocytotoxic and the increase in colony stimulating activity was noted in steroid-treated lymphocytes. This augmented activity might be mediated by suppression of production of the conony stimulating activity inhibitor.22) Moreover, steroids suppress the production of interleukins and interferons by activated T cells, which positively influence hematopoiesis.23)
The simultaneous use of high-dose steroid and ALG induced salt retention, edema, hypertension and transient hyperglycemia. The high-dose steroid was as effective as a lower dose in terms of response rate in aplastic patients when treated with ALG (Table 8).
In view of specificity, ALG reacts with most of the lymphocytes in circulating blood, granulocytes, platelets, the thymus, renal cells, hepatocytes, and breast cells.24) But we could not observe any side effects on these organs. It is not believed that the ALG effect is mainly from the lymphocytotoxic effect because pan-T monoclonal antibody cannot induce the same effect as ALG.10)
Most of the aplastic patients have increased activated suppressor T cells,6,7) by which the helper and suppressor T cell ratio (T4/T8 ratio (is decreased.25) These activated suppressor T-cells produce lymphokines such as gamma-interferon and suppress the hemopoiesis.8) Some authors suggested that the increased interleukin-2 activity in the patient's serum might be a parameter of the disease activity in aplastic anemia.26)
Zoumbos and his colleagues suggested the importance of the role of lymphokines in ALG responding aplastic patients; that is, the decreased activity of gamma-interferon and interleukin-2 could be observed only in ALG responding patients.6) On the contrary, the immunostimulation effect of ALG provides the possible explanation of the ALG effect on hemopoiesis. A small non-cytotoxic concentration of ALG acts as a strong mitogen in vitrc, and induces blastogenesis of lymphocytes, which in turn increases the production of hematopoietic growth factor.27)
The initial reports of clinical19) and in vitro26) studies of cyclosporin in patients with aplastic anemia were not optimistic. In our study, we attempted to remove the activated lymphocytes with ALG and then block the possible recruitment of activation of lymphocytes in aplastic patients by means of maintaining immunosuppression with cyclosporin therapy. Cyclosporin is a non-cytotoxic immunosuppressant which blocks the interleukin-1 and IL-2 secretion,28) reduces the expression of HLA-DR and IL-2 receptors and suppresses the induction of activated T cells and gamma-interferon, which suppress the CFU-GM in vitro.29) These effects of cyclosporin could enforce the effect of ALG. Moreover, the suppression of NK activity by cyclosporin might provide the chance to respond to the combination therapy with ALG and cyclosporin in those who did not respond to ALG alone therapy. Some authors suggested the possible adverse effect of cyclosporin. Cyclosporin alters the protein of ALG and reduces the effect of ALG.19) An in vitro study indicated that the addition of cyclosporin could not increase the CFU-C production.27) Steroid dose, age and sex of the patients could not be a parameter for predicting the response to our ALG plus CSA therapy (Tables 7, 8). The lapse time between initiation of therapy and the beginning of response was three months in half of our patients. Only one of the responding patients responded at twelve months after treatment, and we would recommend following the patients for at least one year after immunosuppressive therapy (Table 6). We also applied the ALG plus CSA regimen to moderate aplastic anemia patients. To our surprise, all nine patients responded in six months (Table 9).
Our data suggest that ALG plus cyclosporin A might be a more useful therapeutic modality for patients with severe aplastic anemia who cannot be candidates for bone marrow transplantation. A randomized multicenter study is needed for confirmation of our preliminary study.