Endobronchial Hamartoma:
A Case Report
Article information
Abstract
Hamartoma is one of the most common benign lung tumors. Most of them are located in the lung parenchyme, but very rarely it can originate endobronchially. We report a case of endobronchial hamartoma in a 59 year old woman and a review of the literature.
INTRODUCTION
Benign tumors of the lung constitute a small minority of all lung tumors but they can cause clinical roentgenographic changes similar to those seen in malignant neoplasms. Hamartoma is the most common benign neoplasm involving the lung. Most hamartomas are located peripherally in the lung parenchyma and endobronchial hamartoma is a rare lesion. Only 6 cases have been reported through 1986 in Korea,1–6) while intrapulmonary hamartoma is relatively common with 19 cases reported in Korea.5,7–11) This paper reviews the rarely encountered endobronchial hamartoma. Though non-malignant, the lesion may cause irreversible lung damage secondary to bronchial obstruction.
CASE
A 59 year old woman was admitted to Seoul National University Hospital because of cough and sputum 14 months before admission, when the cough and fever developed. At that time, she visited an other hospital, where a bronchoscopy was done, and a round rubbery mass obstructing the bronchus intermedius was noticed. Biopsy revealed only chronic nonspecific inflammation. She was well till 2 months prior to admission when the cough developed again. A second bronchoscopy with biospy was done at that hospital with the same pathologic finding, so she was referred to Seoul National University Hospital.
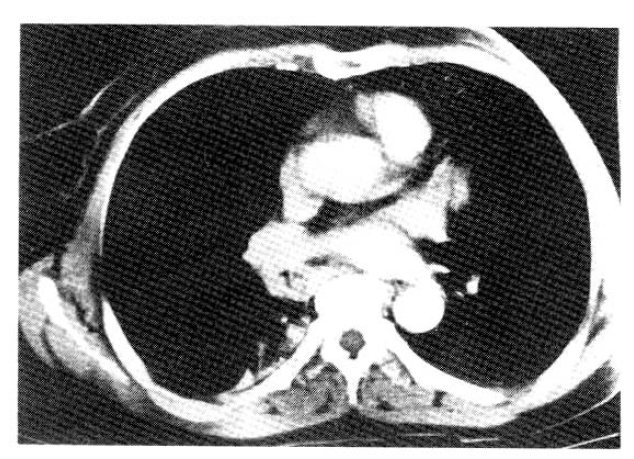
There was no history of tuberculosis and she was a non-smoker. On physical examination body temperature was 36.4°C, the pulse rate was 140/min and the respiration rate was 28/min. Blood pressure was 120/70 mmHg. She appeared dyspneic and no abnormal physical findings were noticed. Hematologic findings were white blood cell count 8,200/mm3 and erythrocyte sedimentation rate 23 mm per hour. Sputum smears for acid fast bacilli were negative and cytology of sputum and bronchial washings revealed no malignant cells. On pulmonary function test, FVC was 2.64 L (108% of predicted value), FEV1 1.24 L, (71% of predicted value) FEV1/FVC 74%. X-ray films of the chest showed right lower lung field collapse, which waxed and waned since March, 1986 (Fig. 1–2). A computed tomographic scan of the chest demonstrated a mass obstructing the bronchus intermedius (Fig. 3). Bronchoscopy and biopsy were done under the impression of bronchial adenoma. The bronchoscopy revealed a movable lobulated pedunculated mass. The pathologic examination suggested squamous cell carcinoma.
An exploratory thoracotomy was performed and just 3 cm distal to the carina, gelatinous endobronchial tumor mass obstructing the right bronchus intermedius was found. Frozen section of the endobronchial mass revealed hamartoma. Right middle and lower lobectomy was done. Her postoperative course was uneventful.
Pathologic findings: On gross examination, in the bronchus to the superior segment of the right lower lobe, a somewhat nodular, polypoid, whitish tan endobronchial lesion was noted and the superior segment of the right lower lobe showed atelectatic consolidation. The mass was a 1.5 × 1.3 × 1.0 cm sized hyalinized ovoid mass occluding the bronchus intermedius, extending to the bronchus of the superior segment of the right lower lobe. The mass consisted of multiple hyaline cartilage islands with ossification, and the cartilage was discrete from the bronchial cartilage plate (Fig. 4). Hyperplasia of the bronchial gland and atypical squamous metaplasia were also noticed.
DISCUSSION
Hamartomas are abnormalities of growth originally described by Albrecht and defined as “tumor-like malformations in which occur only abnormal mixing of the normal components of the organ.”12)
Less than 1% of the tumors of the lung are benign and of these hamartomas are by far the most common.13) McDonald and his associates reported the incidence of hamartoma in the general population to be 0.25%.14) The reported ratio of male to female is 2:1 to 4:1. According to Bateson. 80.5% of pulmonary hamartomas were intrapulmonary and 19.5% were endobronchial.15) Joseph stated 10.3% were endobronchial and Arrigoni said only 3% were endobronchial.16) Worldwide, 58 cases of endobronchial hamartomas were reported until 1972.17)
The histogenesis of hamartoma is unclear. Hodges suggested four etiologic theories; congenital malformation, hyperplasia of normal structure, neoplasia and response to inflammation.18) The theory of congenital origin is accepted by most authors. But recent studies by Bateson support the theory that both forms of hamartoma (intrapulmonary and endobronchial) are similar tumors of primitive bronchial mesenchymal tissue which has the capacity to differentiate toward multiple, mature, mesenchymal components and the only differences being the location and the direction of tumor growth.19) He considers these tumors to be true neoplasms and not congenital malformations.
The endobronchial hamartoma is usually pedunculated and originates from the proximal portion of the bronchial tree.20) 54.5% of endobronchial hamartomas occur on the right side of the lung and 45.5% occur on the left side.21) According to Dovenbarger’s analysis of 28 cases of endobronchial hamartomas, 19.4% of endobronchial hamartomas were located at the left main bronchus, 12.9% at the right main bronchus and 12.9% in the right lower lobe.22)
Several investigators have reported an association of chondromatous hamartoma of the lung with malignant lung neoplasms and possible malignant degeneration of the benign tumor.23,24) A coincidence with extrapulmonary noeplasms was also mentioned, as in Carney’s syndrome which consists of chondromatous hamartoma of the lung, extraadrenal paraganglioma, and gastric leiomyosarcoma.25) Karasik et al suggested that the risk of lung cancer in chondromatous hamartoma of the lung patients was estimated to be 6.3 times higher than the age-sex-ethnic adjusted rate expected for the general Israeli population26) and that no increased risk for malignancies of other sites was found. Anderson reported a case of 2 additional lesions appearing 9 years after primary resection of chondromatous hamartoma.27)
Patients with intrapulmonary lesion are usually free of symptoms. Clinically those patients with an endobronchial lesion are often symptomatic with fever, wheezing or hemoptysis whereas those patients with parenchymal chondromas are not. In this case, the patient complained of recurrent cough sputum and fever.
Most hamartomas are discovered on routine chest roentgenography. Characteristically, the lesion is round, has sharply defined margins and varies in size from a few millimeters to 30 cm in diameter.28) Calcification is present in 10 to 15% of the cases but the pattern of calcification may have no distinctive features.13) However, lamination does not occur, and the “popcorn” pattern is common in hamartomas and rare in other lesions.29) By Dovenbarger, pictures of chest X-ray of the endobronchial hamartomas show atelectasis, lung parenchymal infiltration, and hilar mass which consists of each 50%, 38.5% and 19.2% of endobronchial hamartomas in order, while the pictures of intrapulmonary hamartomas show predominantly a single coin lesion.22)
Definite diagnosis can only be made by histologic examination of the lesion. Rarely adquate tissue can be obtained through a bronchoscope, so usually a thoracotomy is required. Bronchography may also be helpful for diagnosing endobronchial hamartoma. The slow endobronchial growth of this benign tumor results in the bronchographic findings of a smooth obstruction with outward flaring of the involved bronchus. In one reported case, the bronchogram demonstrated a thumb-printing filling defect in the left main bronchus.30)
The usual treatment for hamartomas is surgical removal, especially in view of its potential malignant transformation and because preoperative diagnosis is generally not possible. Most solitary subpleural hamartomas may be removed by enucleation. A wedge resection or a segmental resection can be useful if the tumor has become inflamed and involved contagious structures. Inflammatory involvement of neighbouring structures may necessitate a more radical surgical procedure, sometimes including resection.31) If the lung distal to the obstruction is irreversibly damaged, lobar resection or even pneumonectomy may be indicated.