Interleukin-2 Effect on the Inhibition of Mised Lymphocyte Reaction Induced by Cyclosporin A
Article information
Abstract
Cyclosporin A (CsA) was found to have remarkable immune suppressive properties without the usual significant myelotoxicity and to be a clinically very useful agent to prevent rejection of organ transplantation or bone marrow transplantation graft-versus-host disease in recently. The inhibitory effect of CsA on the production of interleukin-2 (IL-2) has been implicated as a major reason accounting for CsA mediated immunosuppression. But the exact mode of its immunosuppressive effect remains unclear. We studied the influence of CsA and exogenous IL-2 on the proliferative response in allogeneic mixed lymphocyte culture.
The results were as follows:
There is apparent inhibition of proliferative response in mixed lymphocyte reaction (MLR) by CsA in a dose-dependent manner, where optimal concentration is approximately 0.5 ug∼1.0 ug/ml.
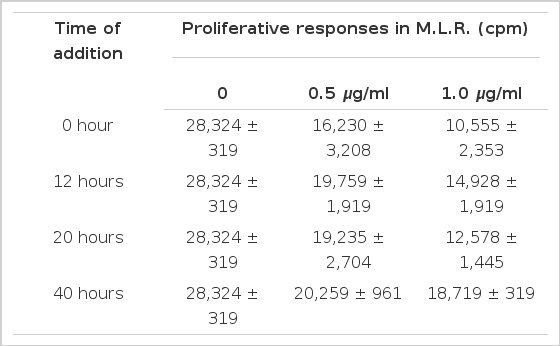
The time-course sequential studies indicated that the lymphocyte response was markedly dependent on the time of addition of CsA to the culture ongoing MLR, the inhibitory effect of proliferative response by CsA was markedly abolished after 20 hours of the culture (Mean cpm 12,578±1,445/28,324±319).
At the beginning of the MLR, an addition of both CsA and exogenous IL-2 (human, 2.0 U/ml) was found to have no appreciable immunosuppressive effect.
Exogenous human IL-2 abolished the CsA effect on the MLR but mouse IL-2 could not.
INTRODUCTION
Interleukin-2 (IL-2) has been known as T cell growth factor (TCGF) Recently, and is a kind of lymphokine concerning T cell proliferation and maturation, and secreated by activated T-helper cell1). Recently, Cs has profound inhibitory activities on lymphocyte mediated II-2 production. Cyclosporin A (CsA) is a biologically active metabolite extracted from two species of fungi imperfecti, Cylindrocarpon lucidum booth and Trichoderma polysporum rifai. Although the potency of CsA as an immuno-suppressive agent has been amply documented both in experimental and in clinical studies, the mechanism of action of the drug is not yet clear. And IL-2 and CsA interaction at the level of T cell were still controversy3,4).
The mixed lymphocyte response is an important in vitro model of the alloimmune response, this study was designed on the influence of CsA and IL-2 by in vitro mixed lymphocyte reaction (MLR).
METERIALS AND METHODS
Powdered CsA was a generous gift from Sandoz, Ltd., Switzerland. The CsA was dissolved in 95% ethanol and diluted to the appropriate concentration in complete culture medium, which consisted of RPMI-1640 (Grand Island Biological Co., N.Y), 20% heat-inactivated fetal calf serum and supplemented with 1% glutamine, penicillin (100 units/ml) and streptomycin (100 ug/ml).
Human peripheral blood lymphocytes were isolated from fresh defibrinated heparinized blood from normal healthy volunteers by Ficoll-Hypaque (Pharmacia Fine Chemicals, N.J.) density centrifugation. The mononuclear cells at the interface were removed and washed three times in RPMI 1640 and then finally resuspended at a concentration of 2.0×106 cells/ml in complete medium.
Exogenous IL-2 could be made from Jurkat-FH CR cells (human lymphoma-leukemia cell line) which were stimulated by phorbol myristrate acetate (PMA) 5 ng/ml and concanavalin-A (Con-A) 20 ug/ml in 5% CO2 incubator for 24 hours5). IL-2 activity could be calculated by proliferation degree of CTLL cells (IL-2 dependent cell line). The procedures of IL-2 assay was briefly described below (Fig. 1). One unit of IL-2 was indicated by half maximal proliferation of CTLL cells. (Fig. 2). Mouse IL-2 could be made from EL-4 cells (mouse tumor cell line) which were cultured and stimulated with concanavaline-A as in same method of human IL-26).

IL-2 unit was quantitiated by half maximal H3-thymidine incorporation of CTLL cells CTLL cell growth kinetics on sereal hilution of crude IL-2, produced by JurkaR-FH-CRC.
Mixed lymphocyte reaction (MLR) were establised using normal human peripheral blood lymphocyts. Briefly, 1×104 responding lymphocytes were co-cultured with an equal number of irradiated (2700 rad137Cs source) stimulating lymphocytes in 96 wells microtiter plate (Limbro®) and incubated for 5 days at 37°C in a 5% CO2 in air incubator. 3H-thymidine (0.5 uCi/well) was added in the microtiter plate and further incubation for 16 or 18 hours. The cells were harvested by cell harvester (Tiertek®) and countered with β-scintillation counter. On bulk mixed lymphocyte reaction, various concentrations of CsA were added on sequential times.
The immunosuppressive effect of CsA on MLR were expressed by percent inhibition of control cpm response.
RESULTS
1. Inhibitory Effect of CsA on MLR.
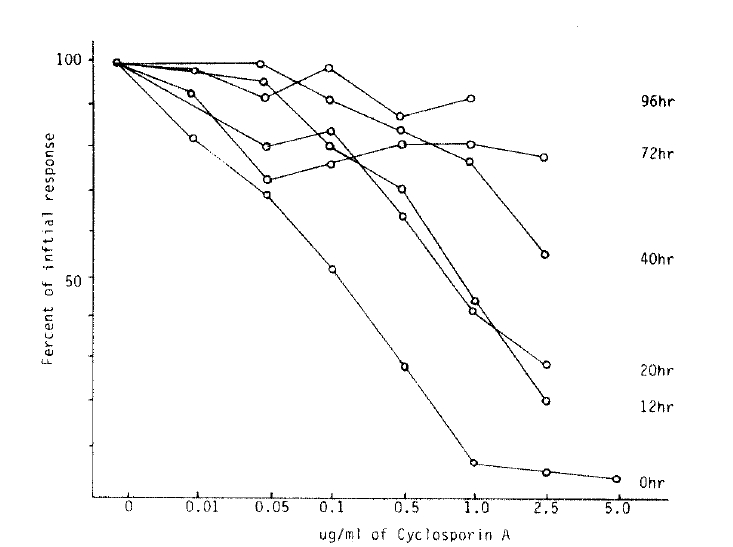
The inhibitory effect of proliferative response according to various concentration of CsA were expressed as percent of initial responses. Adding CsA in graded concentration such as 0.01 ug/ml, 0.05 ug/ml, 0.1 ug/ml, 0.5 ug/ml, 1.0 ug/ml, 0.5 ug/ml, 1.0 ug/ml and 2.5 ug/ml on each well, the mean percent of initial response in MLR showed 87.4±11.6%, 78.2±13.9%, 70.4+14.0%, 40.8+14.8%, 29.3±12.9% and 22.9+11.7%, comparing with that of control mixed lymphocyte proliferative response (mean cpm response was 28.324±329, expressed as 100%). There is more than 50 percent inhibition of proliferative response in MLR by CsA, the optimal concentration is approximately 0.5 ug/ml in each well (Fig. 3).

Percent of inital mixed lymphocyte responses at various concentration of CsA in allogeneic mixed lymphocyte reaction.
This result gave us very useful information deciding optimal immunosuppressive doses of CsA in vivo.
2. Proliferative Effect of CsA Adding after Various Intervals of Ongoing Mixed Lymphocyte Cultures.
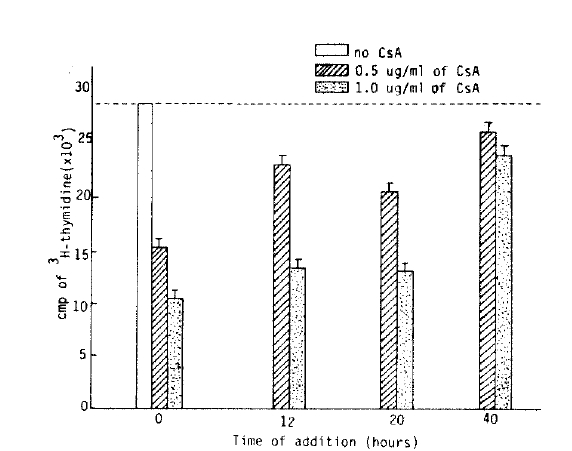
The lymphocyte proliferative responses were studied by a time-sequential addition of CsA in MLR. Adding CsA with in the first 20 hours after initiation of mixed lymphocyte culture, the proliferative response were significantly suppressed in dose dependent manner (Table 1). But we could not observe significant inhibitory effect of CsA offer 40 hours of the culture (Fig. 4, 5). The immunosuppressive effect of CsA was progressively weakened as long as incubation time after initiation mixed lymphocyte culture. These results supported some kinds of products, probably IL-2, on the reaction of mixed lymphocyte culture after 40 hours. Could abolish immunosuppressive effect of CsA7).
3. Influence of Exogenous IL-2 on the Immunosuppressive Effect of CsA in the MLR.
The immunosuppressive effect of CsA was observed in apparently dose dependent manner. Addition to CsA plus IL-2 (Human 2.5 u/ml) at the initiation of the mixed lymphocyte culture, inhibition of the proliferative response by CsA was not observed (Fig. 6). This findings strougly suggested that biological activity of exogenous IL-2 derived from Jurkat-FH-CRC, play a similar role of endogenous products probably produced after 40 hours of MLR.
In contrast to human IL-2, mouse IL-2 (derived from EL-4 cell) could not affect the immunosuppressive effect of CsA. From these results, the immunosuppressive effect of CsA. In lymphocyte proliferative response was almost completely abolished by exogenous human IL-28).
DISCUSSION
Interleukin-2 is a kind of lymphokine (T cell growth factor) and is secreated by activatd T cells (T-helper cells) that as activated by class II antigens and specific antigens, presented by macrophage or non-specific mitogen. IL-2 can influence the proliferation and maturation of resting T cells1). In many immunological diseases, IL-2 could be applied in therapeutic approaches. CsA is a cyclic polypeptide consisting of 11 amino acids extracted from fungus. Because CsA has profound immunosuppressive properties without any significant myelotoxicity, it is widely used in the management of orgen transplantation patients4,2). In a variety of experimental animal systems CsA appeared to be capable of inducing transplantation tolerance. Administration of CsA resulted in the inhibition of antibody formation to T-dependent antigens9), the prolongation of allograft survival in organ transplantation12–14). And the suppression of delayed type hypersensitivity skin reaction. Furthermore, additional studies reported that CsA failed to inhibit the antibody responses to lipopolysaccharid antigens15), suggesting that the action of CsA was predominantly or exclusively limited to T lymphocyte dependent processes1,16).
Recently, CsA evidently acts at an early stage on lymphocyte activation and has no discernible effect on unstimulated cells. Unfortunately, little is known of its mode of action except that in mitogen-stimulated lymphocytes, CsA rapidly inhibits the uptake of uridine, thymidine and amino acids. Several investigators have postulated that CsA is toxic to proliferating lymphocytes leading to an apparent clonal elimination of the antigen reactive T cell pool3,16,17).
If CsA causes functional elimination of the responding clones of cells, then CsA might be a powerful tool for analyzing lymphocyte subpopulations. But little information exists on this points. In vitro, CsA inhibits the induction of cytolytic lymphocytes in primary MLR permitting the activation and expression of suppressor cells, a process that leads to a state of specific alloantigen tolerance that is maintained by a nylon-wool-adherent suppressor cell18–20).
Several investigators reported that CsA suppressed the lymphocyte response to stimulation with mitogen and with alloantigen in MLR in a dose-dependent fashion and addition of CsA to ongoing culture could have the best inhibitory effect on lymphocyte proliferative response10,11). The authors showed similar results in that approach. The best time of significant inhibitory effect of CsA was within 20 hours after culture, and after 40 hours culture, the inhioitory effect of CsA was markedly decreased (Fig. 4).
The authors found that the optimal concentration of CsA which showed more than 50% inhibitory effect was 0.5 ∼ 1.0 ug/ml. It is very important that this quantity of CsA indicates the appropriate effective clinical immunosuppressive dose of CsA in the management of organ transplant patients. The inhibitory effect of CsA may also represent a preferential effect on disrupting the complex set of T cell: T cell interaction known to occur in MLR. The change of inhibitory effect of CsA in time sequences might be that CsA, like many immunosuppressive drugs, acts at a specific stage in the cell cycle, e.g., in G0 with less effect in G1 or later. Dos Reis et al reported that CsA could suppress T cell activation due to the mitogen (concanavalin A) and block IL-2 production by T cell but could not block the induction of IL-2 receptors16). In contrast, the primary T cell proliferative responses to self-la antigens were blocked by CsA and this suppressive effect could not be corrected by addition of exogenous IL-2 receptor on the responding T cell population21). CsA inhibits T cell activation both by blocking IL-2 production as well as by inhibiting the induction of IL-2 responsiveness18,21). Several investigators said that CsA suppressed Con-A induced T cell proliferation by a blockade of IL-2 (TCGF) production and/or secretion and CsA could not have suppressed the T cell that is fully developed IL-2 receptors. These results have demonstrated that the inhibitory effect of CsA was more strong in initial stage of lymphocyte activation but it was markedly decreased in full activated lymphocyte (Fig. 3,4, and Table 1).
CsA effectively inhibits lymphocte proliferation at the initial stage which may explain the ability of CsA to inhibit primary and secondary immune responses. In selected doses, CsA preferentialy inhibits the production of IL-2, probably generated after 40 hours in mixed lymphocyte culture. CsA may not only be a useful drug in clinical transplantation, but become an important immunologic tool allowing the dissection of complex cellular interactions involved in immune responses in vitro and in vivo.
The authors have observed that exogenous IL-2 inhibits the suppressive effects of CsA on MLR8) (Fig. 6). This CsA could restrict production of a kind of lymphokine (blastogenic factor) or inhibit a function of lymphokine. It, therefore, appears that CsA has two primary functions; first, an inhibitory effect on IL-2 production; and second, the ability to inhibit the development of a receptor for IL-2 or at least functional responsiveness by the precursor cytotoxic T cell but apparently not by other lymphocyte subsets.
Recent studies by the authors have demonstrated that human IL-2 blocked the inhibitory effect of CsA but mouse IL-2 did not22).
Furthurmore, authors need more investigation in terms of II-2 receptor expresion, T-T cell interaction and generation of cytolytic effector cells by IL-2.
Notes
This Work was supported by Clinical Research Funds of Catholic Medical Center in 1987.