Morphological Change in Pancreatic Islets, Insulin Binding and Intracellular Glucose Metabolism in Adipocytes of Streptozotocin-induced Diabetic Rats*
Article information
Abstract
To examine the diabetogenic mechanism of streptozotocin, a histological study was performed on the pancreatic islets of male Wistar rats 7 days after an intravenous injection of streptozotocin (75 mg/kg). Furthermore, for the investigation into mechanism of insulin resistance in the insulin-dependent diabetic rats, insulin binding, glucose transport, and lipogenesis were studied in the isolated adipocytes of streptozotocin-induced diabetic rats.
1) The rate of weight gain in the control rats and the diabetic rats were 2.2 ± 1.8 g/day and −2.4 ± 2.1 g/day, respectively (p<0.05). The serum insulin levels in the control rats and the diabetic rats were 23.1 ± 10.8 μU/ml and 16.9 ± 10.6 μU/ml respectively (p<0.05). The plasma glucagon levels in the control rats and the diabetic rats were 165.7 ± 124.9 pg/ml. and 151.2 ± 78.2 pg/ml, respectively (p>0.1).
2) Microscopic examination of the pancreatic islets of the diabetic rats revealed a severe degranulation and disintegration of B cells. Electron microscopic examination showed a disruption of the intracytoplasmic organelles of B cells with complete degranulation. But the morphology of A cells was normal.
3) Maximal specific insulin binding to receptors in the adipocytes of the diabetic rats (2.10 ± 0.30%) showed an increase compared with that of the control rats (1.12 ± 0.21 %) (p<0.05).
4) Insulin receptor concentration in the adipocytes of the diabetic rats (2.33 ± 0.43 ng/0.5 × 105 cells) also showed an increase compared with that of control rats (1.03 ± 0.15 ng/0.5 × 105 cells) (p<0.05).
5) The average affinities in the adipocytes of the diabetic rats slightly decreased in the low receptor occupancy state compared with that of the control rats.
6) Basal and insulin-stimulated glucose transports in adipocytes of the diabetic rats decreased compared with that of the control rats. The maximal glucose transport in the adipocytes of the diabetic rats was 31.7% of the control value (p<0.005).
7) Basal and insulin-stimulated lipogenesis in adipocytes of the diabetic rats decreased compared with that of the control rats. The maximal lipogenesis in the adipocytes of the diabetic rats was 38.4% of the control value (p<0.005).
It can be concluded that streptozotocin produces a diabetogenic mechanism in Wistar rats by directly injuring the pancreatic B cells and inducing hypoinsulinemia. The insulin resistance in the streptozotocin-induced diabetic rats results from the defects in the intracellular glucose metabolism such as glucose transport and lipogenesis.
INTRODUCTION
It is well known that streptozotocin, an antibiotic extracted from Streptomyces acromogenes3), has oncolytic2), oncogenic and diabetogenic properties3). The diabetogenic mechanism of streptozotocin in rodents are direct or indirect damage to pancreatic B cells according to the dose, mode of administration and type of species4–6). Several workers showed that insulin dependent diabetes mellitus accompanies inpaired glucose utilization in liver7,8), muscles9) and adipose tissue10) in spite of the increased number of insulin receptors in vitro. This means that insulin-dependent diabetes has insulin resistance with a high receptor number.
To investigate the diabetogenic action of high dose streptozotocin in rats, we observed the morphology of pancreatic islets of the streptozotocin-induced diabetic rats. To assess the mechanism of insulin resistance in insulin dependent diabetes mellitus insulin binding, glucose transport and lipogenesis were studied in isolated adipocytes from the epididymal fat pad in streptozotocin-induced insulin dependent diabetic rats.
MATERIALS AND METHODS
1. Animals
Male Wistar rats weighing 120 to 190 gm were used throughout the experiments. The animals were kept on a standard pellet diet and tap water ad libitum before experiments.
In order to induce insulin dependent diabetic rats, streptozotocin (75 mg per kg body weight) was injected through the tail vein 7 days prior to use.
2. Materials
Porcine monocomponent insulin was obtained from Novo Ind. (Denmark). [125I]-insulin [SA 250 μ Ci/μg] was purchased from New England Nuclear Co. (Boston, Mass.). D-glucose, bovine serum albumin (fraction V) and collagenase (type II) were purchased from the Sigma Chemical Co. (St. Louis, Mo.). 2-deoxy-D- (1-3H) glucose (SA 22 Ci/mmol) and D- (3-3H) glucose (SA 5.3 Ci/mmol) were purchased from Radiochemical Centre (Amersham, England). Streptozotocin was purchased from Upjohn Co. (Kalamazoo, Mich.).
3. Histology
The pancreas was fixed in Bouin. The staining techniques used were hematoxylin and eosin, aldehyde fuschin and Scott’s method. For electron microscopic examination (JEOL 100B), samples were fixed by immersion in a 1% glutaraldehyde −1.5% paraformaldehyde solution buffered with phosphate (pH 7.2) and post-fixed in a 2% osmic acid. After ultrathin section, the samples were stained with uranyl acetate-lead citrate.
4. Adipocytes Isolation
Isolated fat cells were prepared as described by Rodbell14) and modified by Gliemann15). Briefly the epididymal fat pads from each rat were incubated in vials containing collagenase, bovine serum albumin (20 g/l) and D-glucose (0.05 mmol/l) in Krebs-Ringer bicarbonate. The air in the vials was displaced with oxygen-carbon dioxide (95: 5%), and the vials incubated in a metabolic shaker at 37°C for 90 minutes. The suspension was filtered through a nylon mesh. The fat cells were left to float to the surface and the infranatant was aspirated and replaced with buffer. The number of fat cells was determined by counting the cells in a hemocytometer. The cell suspension was diluted until it contained 0.5 × 105 cells per ml. The viability of fat cells was assessed by the trypan blue dye exclusion test.
5. Binding Studies
Isolated fat cells suspended in a Tris buffer containing bovine serum albumin (30 g/l) were incubated with 125I-insulin (0.2 ng/ml) and various concentrations of unlabeled insulin in plastic vials in a shaking water bath for 90 minutes at 37°c. The incubations were terminated by removing aliquots (200 μl) from the cell suspension and rapidly centrifuging the cells in plastic microtubes to which 100 μl of silicone oil had been added. The cells were then removed and the radioactivity was determined. In these experiments, nonspecific binding was defined as the amount of 125I-insulin remaining in the cell layer in the presence of a large excess (2 × 103 ng/ml) of unlabeled insulin.
6. Glucose Transport Studies
Glucose transport was measured by the method of Olefsky16). To measure the stimulatory effect of insulin to the uptake of 2-deoxyglucose, the cells were preincubated with insulin for 1 hr at 37°C. Isolated adipocytes were incubated with 2-deoxy-D- (1-3H) glucose (SA 22 Ci/mmol) at a concentration of 0.2 μCi/ml in Krebs-Ringer bicarbonate buffer (pH 7.4) containing bovine serum albumin (30 g/l). The assay was terminated at the end of a 3-min incubation at 37°C by transferring 200 μl aliquots from the cell suspension to plastic microcentrifuge tubes containing 100 μl of silicone oil. The incubation was considered terminated at the instant centrifugation began. The radioactivities of the fat cell layers were counted by Packard liquid scintillation spectrometer (TRI-CARB 300 C) after mixing with 100 ml toluene scintillant. Results were expressed as the quantity (DPM: the activity of disintegrating atoms per minute) of 2-deoxyglucose transported to adipocytes (105 cells) under the insulin stimulation in 3 minutes and plotted on the y-axis.
7. Lipogenesis
Lipogenesis from glucose was determined by the method of Moody et al.17). After fat cells had been incubated in a shaking water bath at 37°C for 90 minutes in 1 ml medium containing D-glucose (0.05 mmol/l), 3- (3H)-glucose 0.1 μCi and various insulin concentrations (0–380 ng/ml). (3H)-lipid was extracted into toluene scintillant and counted by Packard liquid scintillation spectrometer. Results were expressed as amounts of glucose (nmol) converted to toluene-extractable lipid in 90 minutes by 105 cells and plotted on the y-axis.
8. Analytical Procedures
Serum glucose was determined using a glucose oxidase method. Serum insulin and plasma glucagon were determined by radioimmunoassay kits (Cambridge; Medical Diagnostics, Inc. U.S.A. and Daiichi Radioisotope Labs. Ltd., respectively).
The values of insulin binding, glucose transport and lipogenesis expressed as means ± S.D. of 3 separate experiments performed on different days. Comparisons were made using Student t-test of statistical significance and differences accepted as significant at the p<0.05 level.
RESULTS
1. Experimental Animals
Table I summarizes the characteristics of the control and streptozotocin-induced diabetic rats. As can be seen, the diabetic group lost rather than gained weight 7 days after the streptozotocin injection. The serum glucose levels on 8th day of streptozotocin injection were markedly elevated in the diabetic group. Serum insulin levels in the diabetic group were significantly decreased when compared with that of the control rats. Plasma glucagon levels of diabetic rats were slightly higher than the levels of control rats.
2. Histology
Examination of sections of pancreas from the diabetic rats stained differentially for islet cells revealed apparently total degranulation of B-cells without cytologic disintegration. But A-cells and acinar cells were normal without any inflammatory cell infiltration (Fig. 6, 7). The ultrastructural study of islet cells revealed the loss of cytoplasmic matrix, the vesiculation of rough endoplasmic reticulum and the disintegration of mitochondria of B-cells. The A-cells were intact (Fig. 8, 9).
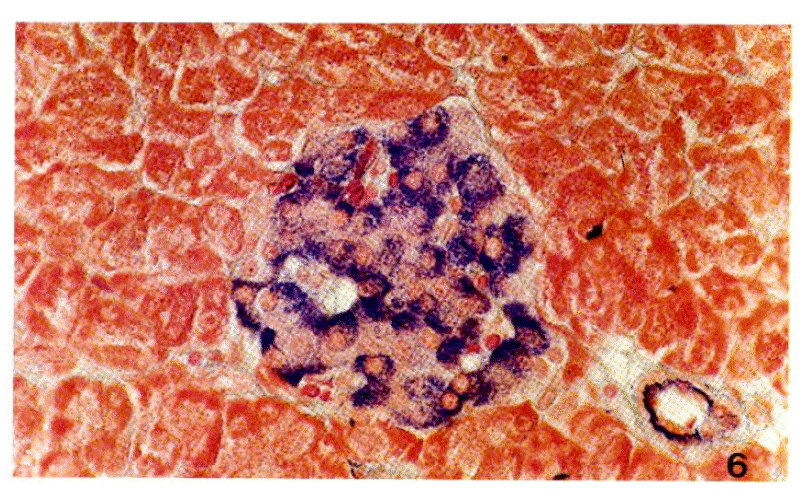
A Langerhoans’ islet from a normal rat.
B cells containing deep blue-colored secretory granules are distributed throughout the islet. A cells containing red colored secretory granules are mainly distributed in the periphery of the islet., Scott’s stain, ×460.
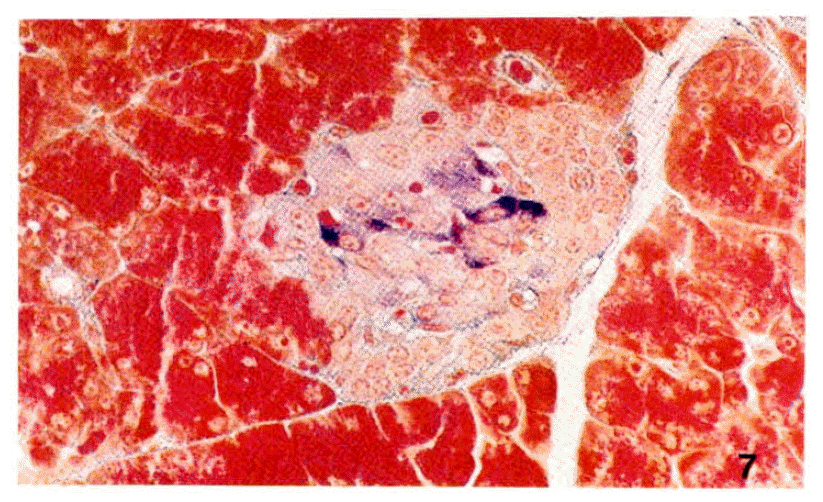
A Langerhans’ islet from a rat treated with streptozotocin (75mg/kg body weight). B cells show remarkable decrease of secretory granules, but A cells are intact X460.

A part of Langerhans’ islet from a normal rat.
B cell (BC) shows well-developed cell organelles such as rough endoplasmic reticulum (r), Golgi apparatus (g) (insect) and mitochondria (m) (insect). Secretory granules (sg) with low to moderate electron density are characterized by wide clear halo enveloped by limiting membrane. A cell (AC) shows granules with a very dark osmiophilic center surrounded by a gray halo ×12,500.
3. Insulin Binding Studies
Adipocytes from diabetic rats bound significantly more insulin at all insulin concentrations except at 380 ng/ml, than those of the controls. The maximal specific binding of insulin to adipocytes from control and diabetic rats was 1.12 ± 0.21% and 2.10 ± 0.30% of the total insulin, respectively (Fig. 1).

Competition-inhibition curves. The percent of total radioactivity specifically bound by adipocytes is plotted against total insulin concentrations (ng/ml).
Values shown are means ± S. D.. Difference between control (closed circle) and streptozotocin-induced diabetic rats (open circle) is significant (P < 0.05).
When these data were analyzed by Scatchard plot18), two curves were approximately parallel suggesting that the increase in binding was predominantly due to a increase in the insulin receptor concentration of the cells (Fig. 2).
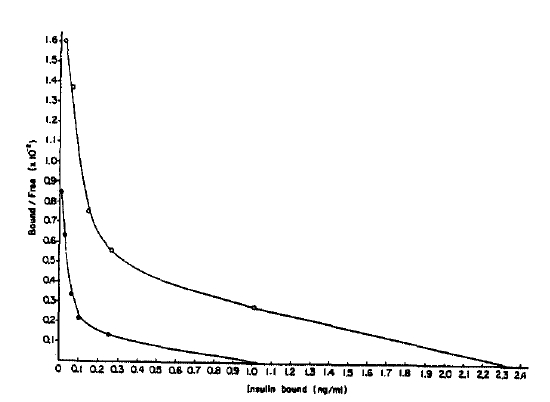
Scatchard plot. Percent bound-to-free insulin is plotted against total insulin bound to adipocytes specifically for the insulin concentrations from 0.2–100ng/ml. The receptor concentrations of controls (closed circle) and streptozotocin-induced diabetic rats (open circle) are the x-intercept of each curve. Values shown are means.
The number of insulin receptors per cell for the diabetic and control rats was 4.8 × 106 and 2.1 × 106, respectively. Average affinity profiles calculated from the Scatchard plot were slightly lower in diabetic rats compared with those of the controls in low receptor occupancy sites (Fig. 3) without any statistical significance19). The average empty site(K̄e) and full site (K̄f) affinities calculated from the average affinity profiles were higher in the diabetic rats (5.5 × 107/M and 1.2 × 107/M, respectively) compared with that of the controls (4.5 × 107/M and 0.8 × 107/M, respectively) (Fig. 2).
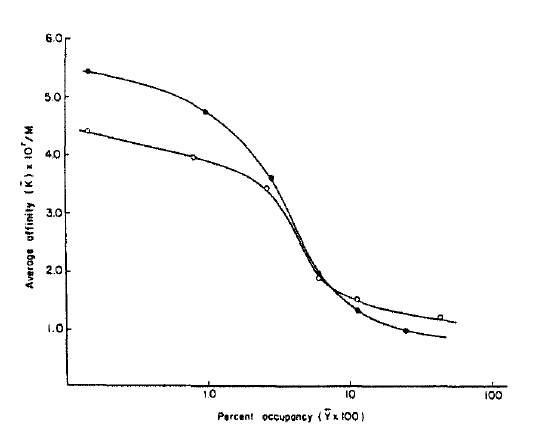
Average affinity profiles of controls (closed circle) and streptozotocin-induced diabetic rats (open circle). The average affinities (K̄) are plotted against the log of the percent occupancy of the receptors (log Ῡ = log B/Ro X 100) at each point. The average empty site affinities (K̄e) are estimated when the value of Ῡ is zero. The average full site affinities (K̄f) are taken as the nadiers in affinity from the affinity profiles (when Ῡ = 1). Values shown are means.
4. Glucose Transport Studies
To evaluate an early step of insulin action in the postreceptor level, glucose transport study was performed. Basal and insulin-stimulated glucose transport activities in adipocytes from diabetic rats were markedly decreased compared with those of the controls. Maximally insulin-stimulated 2-deoxyglucose transport in adipocytes from diabetic rats was decreased to 31.7% of the control value (Fig. 4A).
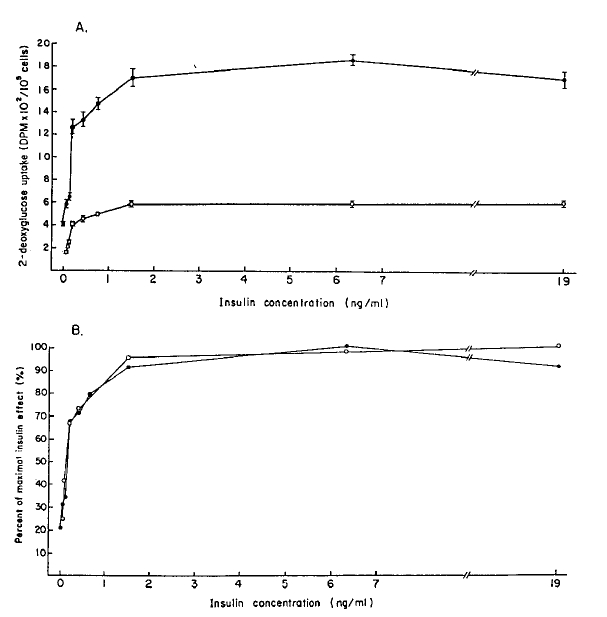
A : 2-deoxyglucose uptake by adipocytes from control (closed circle) and streptozotocin-induced diabetic rats (open circle). The values of 2-deoxyglucose are expressed as DPM (the activity of disintegrating atoms per minute). Data represent means ± S. D..
B : Data in A are plotted as percent of maximal insulin effect. This is calculated by dividing increment in 2-deoxyglucose uptake at each insulin concentration by maximal increment (insulin concentrations of control and diabetic rats are 19ng/ml and 6.3ng/ml respectively). Data shown are means.
To assess the sensitivity of the glucose carrier system, the values at each insulin concentration were expressed as a percent of the maximal insulin effect (Fig. 4B). With this analysis the curve from the diabetic rats was accorded with those of control at all insulin concentration.
5. Lipogenesis
To analyze one major pathway of glucose metabolism in postreceptor level, the rate of glucose conversion to triglycerides was measured in the absence and presence of insulin with adipocytes from control and diabetic rats. Basal and insulin-stimulated lipogenesis in adipocytes from the diabetic rats significantly decreased than those of controls at all insulin concentrations.
The maximal lipogenesis in adipocytes from the diabetic rats was 38.4% of the control value (Fig. 5A). If the data are analyzed as percent increase above basal, insulin-stimulated lipogenesis in adipocytes from diabetic rats is significantly decreased at all insulin concentrations (Fig. 5B).
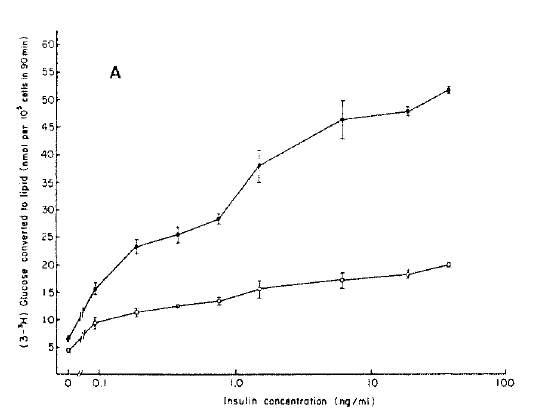
A : Ability of adipocytes from controls (closed circle) and streptozotocin-induced diabetic rats (open circle) to convert the glucose to lipid. Values shown are mean ± S.D.

B : Data in A are plotted as percent increase above basal. Values of lipogenesis in the presence of indicated concentration of insulin were divided by values of lipogenesis in basal state. Values shown are mean.
From those results it can be seen that the activity of lipogenesis in adipocytes from diabetic rats was also markedly suppressed at all insulin concentrations.
DISCUSSION
The diabetogenic mechanisms of streptozotocin in experimental rodents are largely divided into two groups. One mechanism is to damage the B-cell directly20). Secondly, by injecting streptozotocin with dividing the dose for several small amounts, cell-mediated immune reactions directed to B-cells may occur by altering the antigenicity of B-cell membranes due to direct cell toxicity to streptozotocin21,22) or type C virus infection6) in some mouse strains. This study showed that a relatively high dose (75 mg/kg) of streptozotocin induced severe insulin dependent diabetes mellitus in Wistar rats by the mechanism of direct B-cell toxicity. It is accorded with the study of Junod et al.4).
In this study, performed to evaluate the mechanism of insulin resistance of insulin dependent diabetes mellitus, insulin binding in adipocytes from streptozotocin-induced diabetic rats was increased significantly compared with the values of control. In Scatchard analysis, it was demonstrated that the increased insulin binding in diabetic rat is due to an increased receptor number. In this study, the average receptor affinities in diabetic rats decreased in high affinity and low capacity receptor site (K̄e) compared with that of the controls. But the average affinities in low affinity and high capacity receptor site (K̄f) were not different between two groups.
There have been some different reports about the insulin binding studies including receptor number and receptor affinity in experimentally induced insulin dependent diabetes mellitus. Wieringa and Krans13) reported that insulin binding and receptor affinity were increased in streptozotocin-induced diabetic rats. Kobayashi and Olefsky12) have found the same result in adipocytes of diabetic rats. However, Misbin et al.23) and Whittaker et al.24) have reported that ketoacidotic diabetic rats induced by high dose streptozotocin (150 mg/kg) have a decreased insulin receptor number and receptor affinity. It is known that a number of conditions, i.e., acute glucose ingestion25), hypoglycemia26), acidosis27), and fasting28) can alter insulin receptor affinity. The diabetic condition of the animal models used in this study are more severe than those of the animals used in the studies of Wieringa and Krans13) and Kobayashi and Olefsky12, but not as severe as those of Misbin et al.23) and Whittaker et al.24). Therefore, moderately severe insulin-dependent diabetic rats may have dissociated results between the insulin receptor number and the receptor affinity. The cause of the increased insulin binding in adipocytes from diabetic rats is probably due to the hypoinsulinemia. This evidence was reported recently by several studies that the insulin concentration could regulate inversely the number of insulin receptor of target cells29). But another possibility that ketoacidosis induced by severe diabetes mellitus may play a role to increase insulin binding can not be excluded30).
The glucose trasport activities in basal and insulin stimulated states, a first step of insulin action in the postreceptor level, were markedly decreased in adipocytes form the diabetic rats in spite of the increased receptor number. From these results, it is concluded that an uncoupling exists between the two systems in streptozotocin-induced diabetic rats. It is poorly defined about the subsequent step to stimulate the glucose transport after insulin binds to its receptor. The decreased binding affinity in low receptor occupancy states in adipocytes of diabetic rats may play a role in the uncoupling between insulin-receptor complexes and the glucose transport system. However, in view of the decrease of 2-deoxyglucose uptake at the same rate in all insulin concentrations used in the glucose transport study, the decreased binding affinity found only in low receptor occupancy states may not be a contributory factor in the uncoupling between insulin-receptor complexes and the glucose transport system.
It is known that insulin promotes glucose metabolism by the activation of glucose transport system31,32). The mechanism of the activation of glucose transport system by insulin is presumably to (1) accelerate the translocation of the preexisting glucose transport activity form intracellular storage pool to the plasma membrane33) and to (2) increase the basal concentration of the glucose transport system in the intracellular storage pool34). Karnieli et al.34) suggested that the decreased glucose transport of the adipocyte in the animal model of insulin dependent diabetes mellitus could be explained by a decrease in the number of glucose transport systems appearing in the plasma membrane in response to insulin as the consequence of a depletion of these glucose transport systems in the cell’s intracellular pool. To evaluate the sensitivity of the existing glucose transport system to insulin, the data were analyzed as the percent of maximal insulin effect on 2-deoxyglucose uptake11). It suggested that the sensitivity of the existing glucose transport system in adipocytes from the diabetic rats to insulin is not different from that of the controls.
The mechanism of this decreased glucose transport in respect to the increased insulin receptor number in adipocytes from the diabetic rats is not clear. However, because it is known that the production and sensitivity of glucose transport system depends on the insulin level, it seems to be due to the decrease in the level of functional glucose transport system rather than the decreased sensitivity by the chronic hypoinsulinemia.
To further evaluate the insulin action in the postreceptor pathway, lipogenesis, one of the major pathways of glucose metabolism, was measured. Basal and insulin-stimulated lipogenesis in adipocytes from the diabetic rats also markedly decreased compared with that of the controls. The mechansim of the decreased lipogenesis in adipocytes from the diabetic rats is probably due to the low glucose transport activity subsequently and/or the low activity of the enzyme system concerning to the intracellular glucose metabolism.
In conclusion, these results suggested the existence of a dissociation between the insulin sensitivity and the insulin responsiveness in adipocytes from the streptozotocin-induced insulin dependent diabetic rats.
The mechanism of this dissociation is probably due to severe hypoinsulinemia which can provoke the increased insulin receptor number, the decreased production or activation of the glucose transport system and/or the derrangement of the glucose metabolism due to decrease in the enzyme activity of glucose metabolism.
In summary, the diabetogenic mechanism of streptozotocin in the Wistar rat is to directly injure the pancreatic B cells and induce hypoinsulinemia. The cause of insulin resistance in the streptozotocin-induced diabetic rat is a defect in the intracellular glucose metabolism such as glucose transport and lipogenesis probably due to hypoinsulinemia.


