Serum and Urine Soluble HLA Class I Antigen Concentrations are Increased in Patients with Hemorrhagic Fever with Renal Syndrome
Article information
Abstract
Objectives
In order to evaluate the association between the Hantaan virus-induced cellular-immune response and clinical severity in patients with hemorrhagic fever with renal syndrome (HFRS).
Methods
We serially measured the serum (n = 16) and urine (n =6) concentrations of soluble HLA class I antigen (sHLA-I) and clinical powameters in patients with HFRS.
Results
Serum sHLA-I concentrations in patients with HFRS were significantly higher than those in controls throughout all clinical phases (p<0.01). The highly elevated Serum sHLA-I concentrations peaked in the oliguric phase and declined gradually through the phases of HFRS. Serum sHLA-I concentrations in patients with hypotensive episode were higher than in those without the episode (5,585±2,184 vs. 2,389±860ng/ml in oliguric phase, 4.111±1,952 vs. 1,502+592 ng/ml in diuretic phase, p<0.05), and serum sHLA-I levels showed a significant correlation with blood WBC count (r=0.75 in the febrile and hypotensive phase, p<0.01 and serum creatinine concentrations (r=0.64 in the oliguric phase, p<0.01), respectively. Urine sHLA-I levels in the oliguric phase were significantly higher than those in the diuretic phase (390±155 vs. 214±45 ng/mg Cr, p<0.05) and urine sHLA- I levels are associated with severe illness in patients with HFRS. The higher serum sHLA-I are associated with severe illness in patients with HFRS. The persistent elevation of serum sHLA-I during all phases of HFRS might be related to increased production due to prolonged cellular immunologic stimulation by the Hantaan virus rather than decreased excretion of sHLA- I through the kidney.
Conclusion
We suggest that the serum and urine sHLA- I concentrations can be used as a stable and objective parameter for monitoring clinical severity and renal dysfunction in patients with HFRS.
INTRODUCTION
Hemorrhagic fever with renal syndrome (HFRS) is associated with high mortality which is related to a high degree of leukocytosis, thrombocytopenia, systemic hypotension and renal dysfunction1) The main pathophysiologic features of HFRS are the injury to vascular endothelium by direct viral invasion2), which results in systemic hypotension, shock3,4), activation of cytokines5–8) and secondary tissue damage following immune reaction9–14).
It has been suggested that cellular and humoral immune reactions might be involved in the pathogenesis of HFRS. T cell activation has been demonstrated, and the CD4-to-CD8 ratio is decreased as a result of increase in the number of CD8, cytotxic T cells9). Immune complexes were detected in the serum and urine10), in the glomeruli11) and the dermal capillaries from biopsy specimens of petechial lesions12) as deposits, and on the surface of platelets and red blood cells10). Higher concentration of the virus and IgM immune complex titer might be related to poorer prognosis and more severe clinical illness14).
Soluble HLA class I antigens (sHLA- I) were found in the serum, plasma or CSF of healthy and diseased individuals15), but very low to undetectable amounts of the sHLA- I was detected in the urine of healthy individuals16). sHLA- I proteins are present with three different forms with molecular weight approximately 45, 39, and 35 Kd, respectively15). An increase in serum concentrations of sHLA- I has been reported in the case of autoimmune disease, graft versus host disease in bone marrow transplantation, hemodialysis (HD) patients, rejection of renal allograft and cytomegalovirus (CMV) infection17–21). Until now, the clinical significance of the increase in the serum sHLA- I concentrations remained unclear. The elevated sHLA- I may be associated with immune modulation of self and the status of immune response22). In CMV infection, increase in HLA-I expression on endothelial cells corresponds to the percentage of the infected cells after CMV infection, and then increase in serum sHLA- I levels followed23). Furthermore, in a mouse model of ischemic acute tubular necrosis, HLA-I expression increased on the ischemic tubular epithelial cells24). Previous immunohistochemical analysis revealed widespread endothelial distribution of Hantaan viruses in various organs in the patients with HFRS12–14). The manifestations of HFRS might be due to the direct cellular effect of viral infection and virus-induced immune-mediated response. In this study, we measured serum and urine sHLA-I concentrations throughout all phases of HFRS to evaluate their association with the clinical severity and renal dysfunction of patients with HFRS.
PATIENTS AND METHODS
A total of 58 healthy subjects and 16 HFRS patients were studied. In the control group, the mean age was 29 years (range 20–42). In the group of HFRS patients, the mean age was 23 years (range 20–40) and all were male in both groups. HFRS was confirmed by immune adhesion hemagglutinin titers and IgM antibodies by ELISA.
Eight of 16 patients experienced an hypotensive episode (systolic blood pressure less than 90 mmHg and a decrease in blood pressure more than 30mmHg compared with convalescent phase). Six of the 16 partients were dialyzed more than 2 times during their illness; the others were not dialyzed, The clinical course of HFRS was divided into 5 phases on the basis of previous reviews25).
The blood samples were allowed to clot in a sterile pyrogen-free tube (Vacutainer, Becton Dickinson, Rutherfield, N.J., U.S.A) at 37°C for 2 hr and then centrifuged at 2.000g at room temperature for 20min. The urine samples were centrifuged as we did the blood samples. Then, the serum and the urine samples were stored in small aliquots at −70°C until tested. The blood samples of hemodialysis (HD) patients were taken before initiation of HD.
The sHAL- I was measured by ELISA method (sHLA-STAT, Sangstat Co. California). White blood cell (WBC) counts, platelet counts and serum creatinine were measured also at each clinical phase in all patients with HFRS.
The values were reported as mean±standard deviation (SD). The Wilcoxon sign-ranks test was used for comparison between the groups, the Mann-Whitney U test for the comparison within the groups (hypotensive vs. non-hypotensive, and HD vs. non-HD). Simple linear regression was used to examine the relationship between clinical parameters and serum sHLA- I levels, and their association was expressed as Pearson’s correlation coefficient (r).
RESULTS
1. Serum sHLA-I concentrations
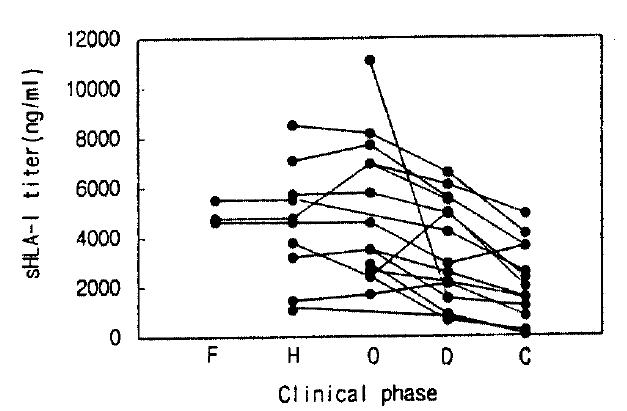
Serum sHLA- I concentrations in HFRS patients showed a wide range (healthy controls 415±256 ng/ml vs. HFRS patients in the febrile, hypotensive, oliguric, diuretic and convalescent phase: 4,963±388, 4,572±2,322a, 5,034±2,191a, 3,312±2,063a, 2,056±1,562ng/ml, respectively) and serum sHLA-I concentrations in each phase of HFRS were higher than in healthy controls, respectively, over the disease phases (all phases were compared with controls, p<0.01, Fig. 1). The elevated sHLA-I concentrations peaked in the oliguric phase, and serum sHLA-I in the febrile, oliguric and diuretic phases were higher than in the convalescent phase (a, p< 0.01, Fig. 1). All patients, regardless of their initial elevation of sHLA-I concentration, showed gradual decline through the phases of HFRS (Fig. 2).
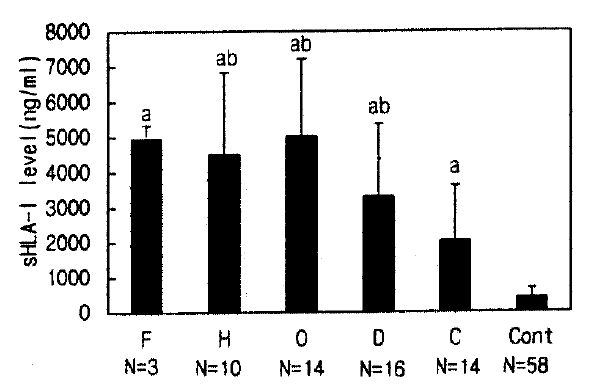
Serum sHLA-I levels of healthy controls and HFRS patients (a: p<0.01 compare with control; ab: p<0.01 compare with convalescent phase; mean±SD, N=patient number). Abbrevation: F: febrile: H: hypotensive: O, oliguric: D, diuretic: C, convalescent phase: Cont, Control.
2. Urine sHLA-I concentrations
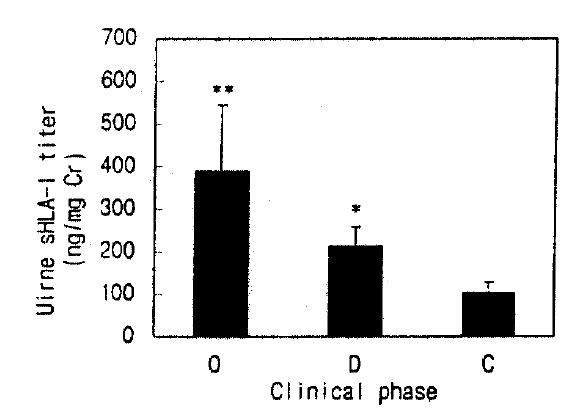
Urine sHLA-I in healthy controls was undetectable. In the patients with HFRS, urine sHLA- I concentrations were significantly higher in the oliguric phase than in the diuretic phase (390±150 vs. 214±45ng/mg creatinine, p<0.05). Urine sHLA-I was elevated during all phases, and was persistently high in the convalescent phase(104±25ng/mg creatinine, Fig. 3).
3. Relationships between serum sHLA-I concentrations and other clinical parameters
Serum sHLA- I concentrations in the hypotensive group were higher than in the non-hypotensive group during the oliguric and diuretic phase, respectively (Fig. 4).
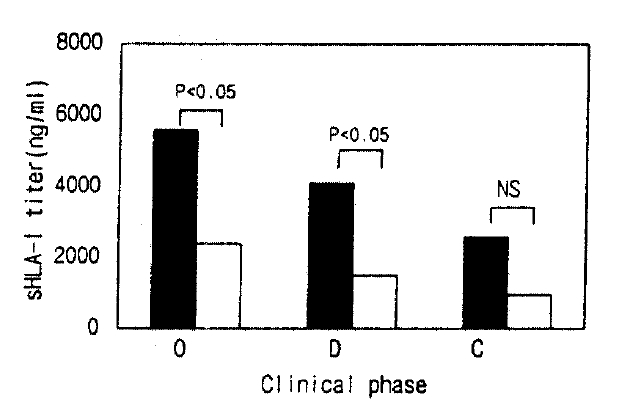
Serum sHLA-I concentrations in hypotensive and non-hypotensive group in the oliguric, diuretic and convalescent phase. The filled bar; hypotensive, the open bar; non-hypotensive group.
Blood WBC counts correlated highly with serum sHLA-I concentrations through the febrile to hypotensive phase (r=0.75, p<0.01, Fig. 5), but there were no correlations between the WBC counts and the serum sHLA- I concentrations in other phases (in the diuretic and convalescent phase, r=0.03, 0.08, respectively). The creatinine levels correlated sensitively with serum sHLA-I concentrations in the oliguric phase (r=0.64, p< 0.01, Fig. 6), but there was no relation to serum sHLA-I concentrations in other phases. The platelet counts did not correlate with serum sHLA- I concentrations throughout all disease phases.

Correlation between serum creatinine and serum sHLA-I concentrations in the oliguric phase with HFRS patients.
DISCUSSION
Our results show that serum sHLA- I concentrations in patients with HFRS are significantly higher than in healthy controls during all phases in HFRS. An earlier and higher increase in the serum sHLA- I concentrations was detected from the febrile phase, and the increased concentration of sHLA- I preceded the elevation of anti-Hantaviral IgM antibody, which showed peak level in the oliguric and diuretic phases26). Considering that high serum sHLA-I concentrations are associated with high urine sHLA- I in the oliguric phase, and low serum sHLA- I concentrations are associated with low urine sHLA- I concentrations in the diuretic phase, it is more likely that the increased serum sHLA- I concentrations are mainly due to incaresed prodution rather than decreased excretion via the kidney. Futhermore, the persistent elevation of serum sHLA- I through all phases, and the elevated urine sHLA- I in the convalescent phase, suggest sustained host reaction to the virus and persistent renal pathology because urine sHLA-I is undetectable or very low in individuals with normal renal function. In terms of molecular weight of the sHLA-I, it may filter the glomerulus, and then would be reabsorbed at the proximal tubule as other proteins of this range of molecular weight20). Previously, Yao et al, demonstrated the presence of Hantaan virus in27)monocytes 3 weeks after the onset of HFRS27), and it might be related to our finding that serum sHLA- I concentrations remained elevated in the convalescent phase. The elevated urine SHLA-I concentrations in the convalescent phase might be due to unsolved renal pathology. These findings suggest that the renal pathology remains for a while in spite of clinical improvement. Regardless of the initial elevation of sHLA-I, sHLA-I concentrations gradually declined in accordance with clinical improvement in all patients.
During the acute phase of HFRS, cytokines, such as interferon-gamma or interleukin-1, complements and hematopoietic growth factors stimulate leukocytosis, and the degree of increased WBC and monocytes in the acute phase has been used as indicators of severe HFRS infection28,29). Our study shows increased sHLA-I concentrations in the acute phase, which is sighificantly correlated with leukocytosis. Because lymphocytes activated by an antigen increase sHLA-I secretion, the elevated serum sHLA-I concentrations during the acute phase suggest that an up-regulation of the HLA-I concentrations in the acute phase may be due to increased lymphocyte counts and secretoty due to increased lymphocyte counts and secretory activity.
Serum sHLA- I concentrations in patients with hypotensive episode are significantly higher than those of non-hypotensive patients in the oliguric and diuretic phase. No difference in serum sHLA-I concentrations was noted between the hypotensive and non-hypotensive groups in the convalescent phase. In the oliguric phase, the worsening of renal function, i.e. increased serum creatinine, also paralleled with increased serum sHLA-I concentrations. These findings suggest that serum sHLA-I concentrations may be associated with the severity of renal failure in HFRS. In view of the close correlation of the elevated serum sHLA I concentrations and renal dysfunction during the oliguric phase, it seems that the kidney might play an active role in the metabolism or degradation of sHLA- I30).
It is well known that the interaction between blood and dialyzer membrane during hemodialysis (HD) stimulates lymphocytes to produce cytokines that increase immune reaction31), so we measured serum sHLA- I in HD and non-HD patients with HFRS in the oliguric phase. Our result shows no difference of sHLA- I concentrations between HD and non-HD patients, This suggests that serum sHLA- I concentrations are not affected by HD in the patients with HFRS. The degree of thrombocytopenia has no relation to serum sHLA- I levels. It might be related that patelets do not secrete sHLA-I but express only membrane-bound HLA- I 32).
In summary, Hantaan viruses activate the cellular-immune system and may allow lymphocytes to secrete sHLA- I. Persistent elevation of serum and urine sHLA- I concentrations during all phases suggests prologed immunologic stimulation. In the febrile, hypotensive and oliguric phase, the increased serum sHLA-I concentrations are due to increased production as well as reduced renal clearance and metabolism. The concentrations of sHLA- I are significantly correlated with the seryerity of renal dysfunction and clinical manifestations, but serum sHLA-I concentrations do not seem to be affected by HD. The detection of urine sHLA- I in patients during the convalescent phase may suggest on-going disease activity and sustained renal dysfunction in spite of clinical and laboratory recovery. In conclusion, the measure of serum and urine sHLA- I concentrations might be used as a stable and objective parameter for monitoring clinical severity and renal dysfunction in patients with HFRS.