Effect of Deamino-8-D-Arginine Desmopressin in Uremic Bleeding
Article information
Abstract
Objectives
This study is designed to evaluate the clinical outcome of uremic bleeding treated with DDAVP. DDAVP was given intravenously in nine ESRD patients who had undergone A/V fistula surgery and showed oozing of the operation site for more than one hour.
Methods
Hemostasis was observed by removing the blood from the wound site with a piece of filter paper for 3 hours after DDAVP administration. vWF, t-PA antigen, t-PA activity, total fibrinolytic activity in euglobulin fraction, fibrinopeptide A and FDP were measured before and after DDAVP administration.
Results
Cessation of the oozing did not occur within 3 hours after DDAVP administration in all of the cases. vWF levels and t-PA antigen were significantly increased after DDAVP administration peaked at 30 min for vWF and 60 min for t-PA antigen. t-PA activity increased in 6 cases and euglobulin fibrinolytic activity increased in 7 cases, respectively. These values fell towards pre-administration levels 120 min after the administration. There was no difference in fibrinopeptide A levels before and after DDAVP administration. FDP became positive in 4 cases after DDAVP administration.
Conclusion
DDAVP increased both vWF and t-PA levels and cessation of the oozing from post-operative AV-fistula wounds did not occur within 3 hours after DDAVP administration in all of the cases. These results suggest that the effect of DDAVP should be reassessed in the treatment of uremic bleeding.
INTRODUCTION
Bleeding is a major contributory factor in the morbidity and mortality of patients with end-stage renal disease1–4). Despite considerable progress in recent years, the pathogenesis of uremic bleeding is still a matter of controversy. However, most investigators agree that uremic bleeding is the result of complex abnormalities involving platelet function5–9), platelet vessel wall interaction10,11), anemia12,13) and drugs affecting platelet function in uremia14).
There have been several therapeutic approaches to the correction of uremic bleeding. Hemodialysis is one of several forms of treatment of uremic bleeding14–16). Uremic patients are often severly anemic, and there is evidence for a relationship between the severity of anemia and the extent of the prolongation of the bleeding time17). Cryoprecipitate infusion was tried with success18). Conjugated estrogen19,20) and DDAVP (desmopressin, 1-deamino-8-D-arginine vasopressin)21,22) were introduced as an alternative to cryoprecipitate in uremic bleeding.
DDAVP induces the release of factor VIII and von Willebrand factor (vWF) from storage site into plasma23). For this reason DDAVP has effectively replaced plasma products in the management of mild haemophilia and von Willebrand disease24,25).
Despite the lack of evidence that uremic bleeding is due to deficiency or abnormality of factor VIII and vWF, the similar biological effects of DDAVP and cryoprecipitate on these haemostatic proteins led some investigators to postulate the DDAVP, which avoids the hazard of disease transmission, might also be therapeutically effective26).
The observation that DDAVP is effective in uremic bleeding dates from the early years of the last decade21,22), but the method of observation in these studies is open to criticism because they observed only the change of bleeding time instead of uremic bleeding itself. We have experienced that the result of bleeding time is not reproducible often in patients with endstage renal disease (data is not presented).
This study is designed to evaluate the efficacy of DDAVP on uremic bleeding objectively from an A/V fistula wound.
PATIENTS AND METHODS
1. Patients
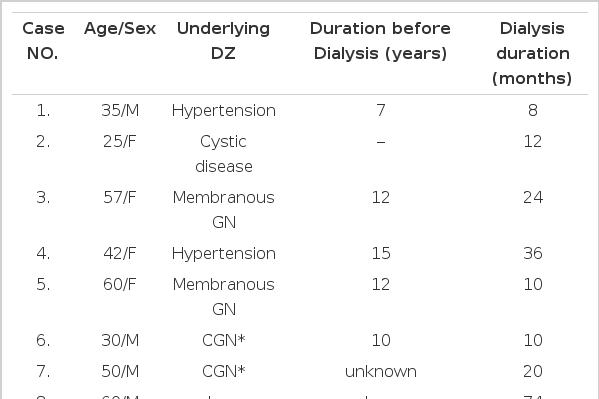
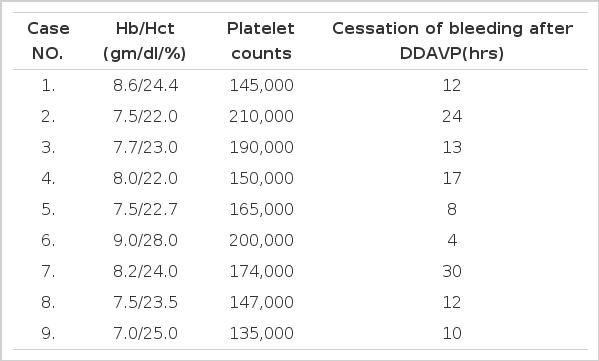
Nine ESRD patients (five males and four females with a median age of 48 years, range 34–60 years) who were attending the hemodialysis unit of Soonchunhyang Chunan hospital, Korea, between January and December 1994, were enrolled in this study. They were fully informed of the details of the study. Individual age, duration of dialysis treatment and underlying disease are shown in Table 1. These patients were admitted for re-operation of arteriovenous (A/V) fistula, because blood flow was not adequate for dialysis. They had normal values in coagulation screening tests (fibrinogen, prothrombin time, activated partial thromboplastin time and thrombin time). Platelet counts were higher than 120×103 per mm3(data not shown). No one had been treated with erythropoietin, aspirin or other drugs that affect platelet behavior for at least 2 weeks prior to the operation. In order to avoid the effect of anemia on hemostasis, hematocrit was corrected above 20% with packed RBC and the mean Hb and Hct values were 7.9±0.6 gm/dl and 23.8±1.8% before surgery (Table 2). All patients were on regular hemodialysis, through the subclavian catheter, 12 hours per week using a cellulose acetate hollow fiber.
The rate of blood flow was more than 200 ml per miuntes, and the rate of water flow was 500 ml per minute.
2. Creating the Fistula
The fistula was created in the dominant arm because it is our policy that the first fistula be created on the noncounter arm. The A/V fistula consisted of a subcutaneous anastomosis of the radial artery to the cephalic vein at the level of the forearm in all of the cases. The surgery was performed in the operating room under regional anesthesia by the same surgeon. The mean length of incision was 3 cm (range; 2.8–3.2 cm). Active bleeding was controlled during the surgery.
Cases showing oozing for more than one hour post-operatively were selected for DDAVP administration.
Desmopressin acetate (Minirin/DDAVP; Desmopressina acetato idrato) was given intravenously at a dose of 0.4 μg/kg body weight, diluted in saline solution and injected over a period of 30 minutes.
Blood samples were obtained by venipuncture, just before and every 30 minutes immediately after DDAVP administration for 120 minutes. Anticoagulant used for analysis of vWF, t-PA activity, t-PA ag, total fibrinolytic activity in euglobulin fraction, Fibrinopeptide A was 12.9 mmol sodium citrate. For t-PA activity assay, 0.5 mL of blood was mixed with 0.25 mL of acetate (20%). Plasma was prepared by centrifugation (3.00 xg for 20 minutes) and kept at −70°C until tested.
The hemostasis was observed directly by removing the blood from the wound site with a piece of filter paper for 3 hours after DDAVP administration. After 3 hours observation, the wound site was gently compressed with gauze which was changed every two hours to confirm the cessation of oozing.
vWF was measured by commercially avaliable ELISA kit (IMUBIND vWF Elisa kit product #828, American Diagnostica inc.).
t-PA activity was measured by commercially avaliable assay kit (Spectrolyse/fibrin, BIOPOOL AB, Cat. No. 101101, Sweden).
t-PA ag was measured by commercially available enzyme-linked immunosorbent assay kit (ASSERACHROM t-PA of DIAGNOSTICA STAGO).
Total fibrinolytic activity in dextran sulfate euglobulin fraction was measured on a plasminogen rich fibrin plate. To make the dextran sulfate euglobulin fraction, plasma (0.5 ml) was diluted with 4.0 ml cold distilled water and a 0.5 ml dextran sulfate solution was added. It was mixed, placed on ice and, with a constant stirring motion, the pH was adjusted to 5.9 (5.85–5.9) with an acetic acid solution. It was left to stand on ice for 30–60 minutes, then centrifuged at 2,000 g for 10 minutes. The precipitate was dissolved in a 0.5 ml saline barbital buffer. 30 μl of this euglobulin fraction was placed on a well controlled fibrin plate. After incubation of the plates at 37°C for 18 hours on carefully leveled shelves in an incubator, two perpendicular diameters of each lysed zone were determined. The mean of the diameters was taken as the diameter of the zone and converted to blood activator unit (BAU) from the reference zone of normal pooled plasma.
Fibrinopeptide A was measured by commercially available enzyme immunoassay of Fibrinopeptide A kit (ASSERACHROM FPA, DIAGNOSTICA STAGO France, Cat. No. 0411).
FDP was measured by latex agglutination method (SPLI-PREST, DIAGNOSTICA STAGO France, Cat. No. 0538).
3. Statistical Methods
Mean values±SD are given in the text. Statistical evaluation was carried out using Wilcoxon rank test through the soft ware stat view 512+(Brain power, calbasa, Calif., USA) operating on a Macintosh PC. Statistical significance was considered to be present if p<0.05.
RESULTS
Cessation of the oozing did not occur within 3 hours after DDAVP administration in all of the cases. It was followed by 12 hours in 5 cases and 30 hours in 4 cases (Table 2).
Fibrinopeptide A levels were 2.9±0.4 ng/ml (range 2.4–3.4) before DDAVP administration and 3.0±0.2 ng/ml (range 2.6–3.4) after DDAVP administration (Table 3).

Changes in Fibrinopeptide A and Fibrinogen degradation product levels before and one hour after DDAVP administration
In all of the patients, FDP was negative before DDAVP administration but positive in 4 cases(case NO; 3, 4, 5, 7) after DDAVP administration (Table 3).
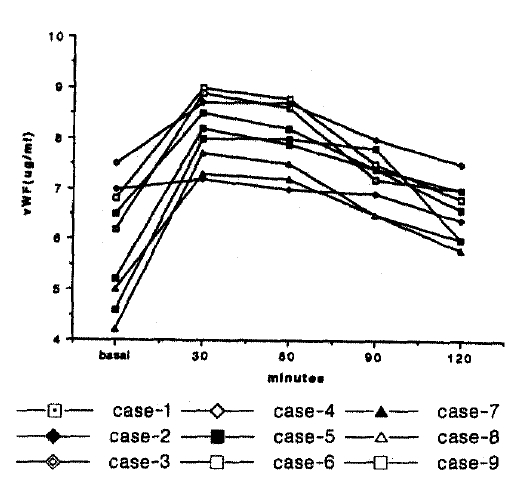
vWF level was significantly increased from 5.9±1.2 μg/ml(range; 4.2–7.5 μg/ml) to 8.3±0.6 μg/ml (range; 7.3–9.0 μg/ml) (p<0.01) before and 30 min following DDAVP administration, respectively (Fig. 1).
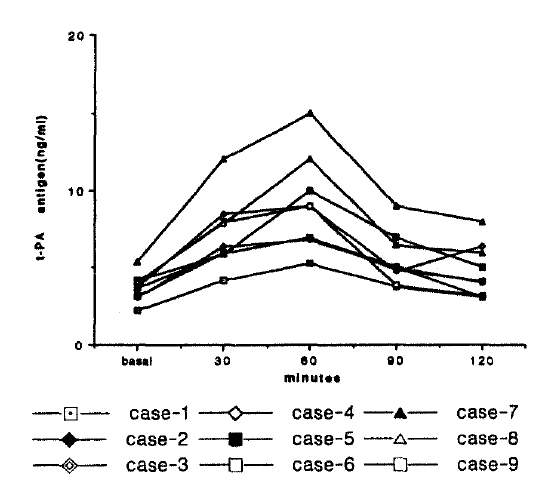
t-PA antigen level was significantly increased after DDAVP administration, from 3,7±0.8 μg/ml (range; 2.3–5.4 μg/ml) to 9.5±3.9 μg/ml(range; 5.3–15 μg/ml) (p<0.01) before and 60 min following DDAVP administration, respectively (Fig. 2).
t-AP activity increased in 6 cases (case NO.; 3, 4, 5, 7, 8, 9) and peaked between 60 min and 90 min after DDAVP administration (Fig. 3).
Euglobulin Fibrinolytic Activity increased in 7 cases (all except case No; 1, 2) and peaked between 60 min and 90 min (Fig. 4).
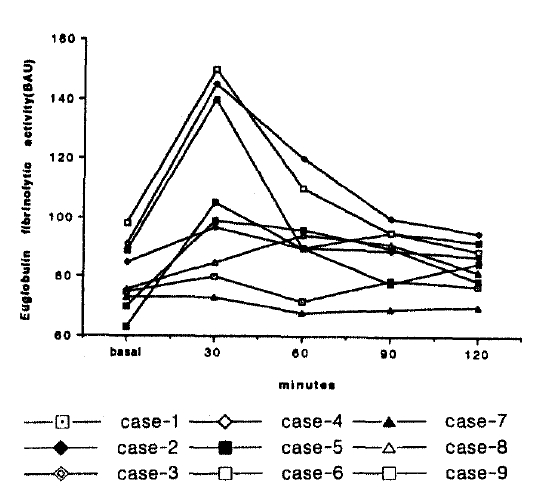
Individual changes of fibrinolytic activity in euglobulin fraction before and after DDAVP administration.
These values fell towards pre-infusion levels 120 min after the administration.
DISCUSSION
The common clinical manifestations of uremic bleeding are ecchymosis, epistaxis, bleeding from gums and venipuncture site, vaginal bleeding and gastrointestinal bleeding. Also, less commonly, hemorrhagic pericarditis, hemorrhagic pleural effusion, subdural hematoma and spontaneous retroperitoneal bleeding have been reported.
Hemostasis in a small superficial wound, such as that produced when measuring the bleeding time, depends on the rate at which a stable plug is formed. It does not, however, discriminate between vascular defects, thrombocytopenia and platelet dysfunction27). The method of the measurement of bleeding time has been developed to imporve the reproducibility, but no one is yet satisfactory. Usually, 40 mmHg pressure was maintained and blood was removed from the wound with a piece of filter paper during the whole procedure of the assay of bleeding time27). This condition is not physiologic because, if venous pressure increased, the rheology which influences hemostasis should be changed. For lack of reproducibility in our preliminary study, we did not measure bleeding time in this study.
To evaluate the efficacy of DDAVP in uremic bleeding objectively, it is very important to know exactly when the cessation of bleeding occurs after DDAVP administration. When blood oozes from an A/V fistula wound, it provides us with a truly standardized wound to objectively observe the exact cessation time of bleeding at the bed side. Besides the convenience of observation of hemostasis, it has the advantage of providing homogeneity of each condition including site, freshness of the wound and characteristics of bleeding. If hemostasis had been induced by DDAVP, it would occur in 2–3 hours becasue vWF reaches the peak levels by 60 min after DDAVP administration at the dose of 0.4 μg/kg body weight28). It is not clear whether platelet adhesion and/or platelet aggregation is always followed by coagulation which yields fibrin formation. In our results, Fibrinopeptide A did not increase and hemostasis did not occur until 3 hours after DDAVP administration.
A variety of endothelial cell agonists, including bradykinin, thrombin, histamine and DDAVP have been shown to cause a simultaneous acute release of both t-PA and vWF28–32). Under physiologic conditions, the activity of coagulation and fibrinolysis are on balance and thrombus formation and/or fibrinolysis occur when one overcomes the other33). In this regard, DDAVP has a two directional activity consisting of both coagulation and fibrinolysis and, even if DDAVP enhances coagulation activity through the release of vWF, it may be counteracted by the simultaneous release of t-PA.
In agreement with previous reports28–31), t-PA antigen, t-PA activity and fibrinolytic activity in euglobulin fraction increased after DDAVP administration. FDP increased concomitantly with t-PA activity in 4 cases. We do not know why the increased t-PA activity was not positively associated with FDP levels in cases NO 8, 9. Perhaps the FDP level may be under the level of the sensitivity of the assay system. These findings suggest that fibrin formation did not occur by DDAVP administration or that concomitantly increased fibrinolytic activitiy counteracts hemostasis activity potentiated by DDAVP.
In conclusion, DDAVP's rise in vWF is not a guarantee for reducing bleeding from post-operative AV-fistula wounds in uremics. Further controlled study is necessary to reassess the effect of DDAVP in treatment of uremic bleeding.