Effects of Endoscopic Variceal Ligation in Lower Esophageal Motor Function: A Prospective Study
Article information
Abstract
Objectives:
Endoscopic variceal ligation (EVL), a recently developed method for controlling active variceal bleeding and eradicating esophageal varices, has similar efficacy to endoscopic injection sclerotherapy (EIS) and is known to have a minimal risk of complications and fewer complications in the lower esophagus. However, since the site of EVL is chiefly done in the lower esophagus, we prospectively evaluated to investigate the effect of EVL on the lower esophageal motor function.
Methods:
We evaluated the severity of esophageal varix with the endoscopy and the lower esophageal manometry in 27 patients who had no history of interventional therapy, for varices before EVL, 3 weeks and 6 months after the last EVL session.
Results:
The EVL caused considerable diminution in the size of esophageal varix by a mean 8.2 (range 3–21) ligations in mean 1.7 (range 1–3) sessions. in most of the cases, the varices reappeared and enlarged when the procedure of EVL was stopped. There were two different types of changes (intermediate and late) in the lower esophageal motility. The intermediate post-EVL effects were the increase of peristaltic contraction amplitude and duration in the lower esophageal body after EVL. The late post-EVL effects were the prolongation of lower esophageal sphincter (LES) relaxation duration and speedier peristaltic velocity in the lower esophageal body.
Conclusions:
We conclude from these findings that the intermediate post-EVL effect may be transient and the increase of peristaltic wave was due to diminution of esophageal varix.
INTRODUCTION
One of the major sequelae in cirrhosis of the liver is esophageal variceal bleeding due to portal hypertension. The effective therapeutic modalities, those used with endoscopy for treating and eradicating acutely bleeding esophageal varices, are endoscopic injection sclerotherapy (EIS) and endoscopic variceal ligation (EVL)1). But, EIS is not a benign process and has many complications2–7), and one of those is the change in esophageal motor function8–11).
A recently developed method that uses small rubber bands for treating variceal bleeding, EVL, is similar to the technique of EIS, and the technique is easy and simple, has a lower complication rate, and the results with EVL are as effective as EIS for controlling esophageal variceal bleeding, so it is recommended as a new desirable therapeutic modality12–17). But there are few reports about lower esophageal complications.
The method of EVL sucks the protruded superficial esophageal varix, then the varices are mechanically ligated and Strangulated, and the histological changes are limited to esophageal mucosa and submucosa. So, there are no disturbances in esophageal motility18,19). Since there are few prospective studies done on it20), we designed this study to investigate the effect of EVL on lower esophageal motor function.
MATERIALS AND METHODS
1. Subjects
We selected 27 (male:25, female:2) patients who agreed to participate in our prospective study. All of these patients [33–66 years of age (mean=51.4±9.4yr)] had esophageal varix due to cirrhosis of the liver (alcoholic: 15, postnecrotic:12) and had a past history of esophageal variceal bleeding, such as hemocyst on the esophageal varix. All of them had no previous endoscopic or surgical therapy. Their clinical features were classified by modified Child’s A; 3, B; 15, C; 9 cases and their varix grade was classified by Paquets21) II ; 2, III ; 11 and IV ; 14 cases.
2. Endoscopic Variceal Ligations
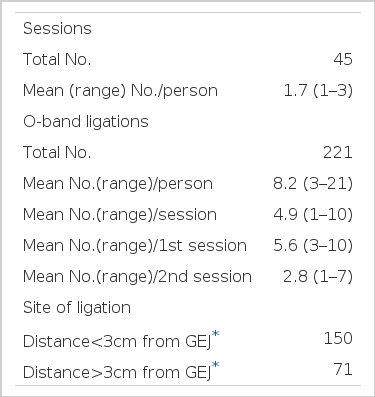
EVL was performed with a Pentax GIF EG 2900 fiberoptic endoscope that passed through a flexible overtube. The basic technique of EVL that we used has been described in other research12). We briefly describe here the technique. The ligating device (Stiegmann-Goff TM, Bard Inc, Bilerica MA, USA) was screwed to the tip of the endoscope, and the trip wire was passed through the biophageal junction. At the initial session, 3–10 ligations were placed, and in the subsequent session, 1–7 ligations were placed, depending on the size and number of the persisting variceal channels. Repeated EVL sessions were done 7–10 days after the varices in the distal esophagus were eradicated or reduced to more than a difference of two grades and loss of variceal bleeding risk signs such as the red-color sign. The follow-up endoscope was evaluated 1 week and 6 months after the last EVL, further ligations being done as needed.
3. Esophageal Manometry
Esophageal manometry was performed at 1–3 days before the first EVL and 3 weeks and 6 months after the last EVL session. The lower esophageal manometry was done with ESM3R (Arndorfer, Greendale, WI) catheter that was passed by mouth into the esophagus, with no anesthesia and sedation, and calculated with the standard method which was reported. The station and rapid pull-through techniques were done to study LES: the lower body was studied by assessing the response to 10 wet swallows (WS: water; 5ml, 20°C). The catheter was perfused with a low-compliance arndorfer capillary infusion system (0.5ml/min) and was connected to an external transducer and recording was traced on a multichannel polygraph (Sensor medics Dynograph Recorder 612, Anaheim, Co, USA). LES pressure was calculated from the mean of three rapid pull-throughs from end-expiratory gastric pressure to end-expiratory sphincteric pressure. The LES length in station pull-throughs was measured on the difference between intragastric pressure in the end-inspiratory and the mean intra esophageal pressure. The duration and percentage of LES relaxation was calculated for three WS when four of the catheter ports were located at the maximal high pressure zone in the distal esophagus. The amplitude and duration to each peristaltic wave was measured at 3 cm above the LES with 10 WS. The speed of lower esophageal peristaltic wave was calculated by measuring the distance between wave peaks over the segment of esophagus from 3 to 8 cm from the upper LES. In all of the subjects, manometry was done with restriction of medicine, starting 3 days before, and no smoking and alcohol 12 hours before. The each manometry was performed in the fasting state and every manometry was done during 9:00–10:00 AM.
4. Statistical Analysis
Results are expressed as means SD. The statistical analysis of the data was carried out by student’s paired t-test by means of a Statview+ 512 program. The p values less than 0.05 were regarded as significant.
RESULTS
The overview of 27 patients who underwent EVL is summarized in Table 1. As scheduled, all 27 patients underwent 1–3 sessions of EVL and second esophageal motility evaluation done postoperatively at 21 days. There had been 21 patients at first, 2 of them died with hepatic failure, 4 of them refused EVL and 1 of them died with infection.
With only 14 patients, a third evaluation was done at 6 months after the last EVL. When follow-up endoscopy was done at one week after the last EVL session, the grade of varix was considered as 2; completely eradicated, 23: grade 1, 2: grade II. At 6 months after the last EVL session, the grade of varix were considered as 6: grade 0 to I, 7;grade II, 1; grade III. The procedures were tolerated well, and no serious side effects occurred. In the first EVL session, 21 patients complained of mild chest discomfort and dysphagia during or after the procedure. The complications such as epigastralgia (2), nausea (1) and bradycardia (1) developed, but they spontaneously subsided within 3 days. At the end of 3 weeks and 6 months, there were no complaints of dysphagia and no stricture developed.
Manometry of Lower Esophagus
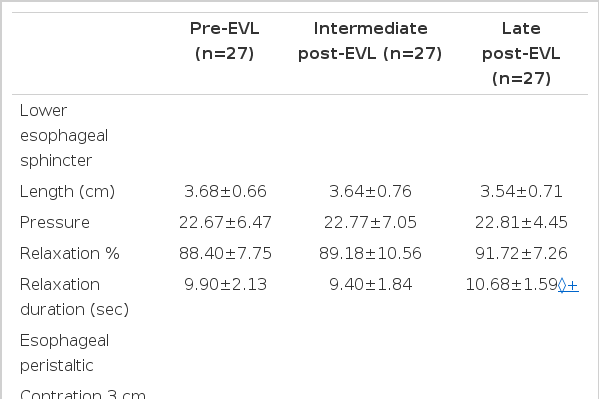
The results of the manometry are summarized as Table 2.
-
Lower esophageal sphincter
No significant difference in LES length, resting LES pressure and LES relaxation percent was found from Pre-EVL to intermediate and late post-EVL. Even though there was no significant change in LES relaxation time at 3 week after post-EVL from pre-EVL, significant prolongation of it was developed 6 months after EVL.
-
Peristalsis of the lower esophageal body
The amplitude of peristaltic wave and contraction duration in the lower esophagus significantly increased after 3 weeks post-EVL, but returned to pre-EVL level at 6 months after the last EVL session. The velocity of peristaltic wave in lower esophagus showed no significant difference at 3 weeks after EVL, but there was significantly increased velocity of peristalsis after 6 months after EVL.
DISCUSSION
EVL is an effective alternative method for controlling variceal bleeding with minimal complications, compared to EIS. Also there are few studies on EVL effects on esophageal motility2,18). This study was designed to compare the effects of EVL on the lower esophageal motility in patients who had undergone variceal ligation.
Our manometric results after EVL in cirrhotic patients with esophageal varices show that, when compared with pre-EVL in the same patients, the effects of EVL in lowere sophageal motility can be divided into intermediate effects and late effects. The former effects are characterized by 1) the amplitude of the peristaltic wave that is significantly increased in the lower esophagus and 2) longer contraction duration of peristaltic wave in lower esophageal body. The latter effects are characterized by 1) longer LES relaxation duration and 2) speedier peristaltic wave progression. Discordant results have been published regarding esophageal motility of EVL effect19). Although we could not exclude these differences, they are due to differences in observation period and methods (3 weeks, 6 months vs mean 8, 9 weeks), subjects (same patients vs different subjects), any possible site of EVL (chiefly LES portion vs no mention) and number of ligators which was used. The intermediate effects of post-EVL in lower esophageal peristaltic amplitude of our results could be supported by other authors19,22) and possible explanations of these finding were proposed by Passaretti et al. They hypothesized that the presence of a ‘cushion’ of blood within the varices might have a damping effect on pressure wave, leading to a decrease in the amplitude and to an apparent increase in duration of contraction. This hypothesis can be supported by our results that lower esophageal peristaltic amplitude increased at intermediate post-EVL as the size of varix diminishes, and then the pressure decreased after late post-EVL as the size of varix diminishes, and then the pressure decreased after late post-EVL when the size of it enlarged22). But there is a discrepancy that does not support the hypothesis about the peristaltic duration in the lower esophageal body. Both peristaltic pressure and duration were similar to the level of those of pre-EVL and late post-EVL.
Also it is known that there is a continuing inflammatory response in the submucosa with maturing scar tissue at 3 weeks, and complete healing process in 50–60 days after EVL18). The prolongation of the duration of peristalsis of intermediate post-EVL might be related to some inflammatory reaction in the submucosa as an acute effect of EIS9).
In the long-term effect of post-EVL, even though there were no demonstrable changes in the percentage of LES relaxation after deglutitiion, there is no relationship between prolongation of LES relaxation time in our results in those when the site of variceal ligation was done chiefly within 3 cm from the gastroesophageal junction, healing process of submucosal fibrosis which appear in 50–60 days after EVL and the esophageal stricture after EVL in lower esophagus. There was some technical faults, even though there were no reliable clinical complications over 6 months. At present, it remains unclear why the duration of it was so prolonged.
Also, the late effects of post-EVL could not explain the increase of peristaltic velocity in the lower esophagus, which still remains unclear now. But a possible suggestion is that the speed of peristalsis is faster in cirrhotic patients with esophageal varix than without any varix and after EIS9,22,23).
Following EIS, there have been reports of variable ranges of esophageal motor abnormalities8–11,23–26). These variabilities may explain the various factors for the study. Nevertheless, there is general agreement that one of the variabilities is in different schedules of motility study after EIS9,11). Like this there is some difference in EVL effects with others, and our results might be related to different schedules, methods and subjects of study.
Even the intermediate EVL-induced motility changes continue over 3 weeks, at least, after the last EVL session. It appeats to be reversible and tends to return to basal motility. We speculate that this dysmotility is caused by submucosal inflammatory changes, injured nerve plexuses and possible muscular fibers due to EVL of those chiefly done in LES region. But the late EVL-induced dysmotility might start before 6 months after the last EVL session, and be permanent. We speculate that this dysmotility is caused by submucosal fibrosis, disturbed nerve plexus and possible fibrosis of muscle due to EVL.
Acknowledgements
The authors thanks Dr. Yong Ho Nah for his technical assistance in collecting the data for this project and Dr. Nam Jin Park for assistance with this manuscript.