Heat Shock Protein Expression in Adenosine Triphosphate Depleted Renal Epithelial Cells
Article information
Abstract
Background
In this study, the putative interactions between apoptosis and heat shock proteins disturbed as a result of ATP depletion were investigated as a hypoxia model.
Methods
The direct cellular damages were assessed by the release of LDH from the cytoplasm of the human tubular epithelial cells (HK-2 cells) following ATP depletion. The Bcl-2/Bax mRNA expression ratio, used as an index to assess to what extent apoptosis contributed to tubular cell damage, and the expressions of HSP 90, 72 and 27 in relation to the Bcl-2/Bax ratio in the ischemic model, as parameters of their functional contributions to tubule cell damage, were also studied. Heat preconditioning (HS) was performed at 43°C in a temperature-regulated water bath for 1 h.
Results
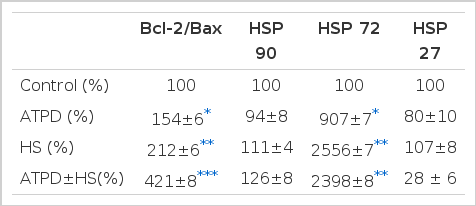
The release of LDH due to ATP depletion was not significantly increased in HK-2 cells compared to the control, but was slightly increased in heat preconditioned cells compared to non heat preconditioned cells, but the difference was not statistically significant (6.33±0.57 U/L vs. 8.67±2.52 U/L, p>0.05). The Bcl-2/ Bax mRNA expression ratio increased progressively from the control to the heat preconditioned and ATP depleted cells (control; 100%, ATP depletion; 154±6%, heat preconditioning; 212±6%, heat preconditioning and ATP depletion; 421±8%). No contribution of heat preconditioning and ATP depletion was observed on the expressions of HSP90 and HSP27. However, HSP72 expression was prominent by ATP depletion, especially after heat preconditioning.
Conclusion
There may be a possibility that the preservation of cytolytic damage and an increase in the Bcl-2/Bax mRNA expression ratio is related to the increase of HSP72 in ATP depletion as a hypoxia model.
INTRODUCTION
Acute renal failure (ARF) is a syndrome characterized by a rapid decline in renal function, retention of nitrogenous waste products and perturbation of the extracellular fluid volume and electrolyte and acid-base homeostasis. It complicates 5 and up to 30 percent of hospital and intensive care unit admissions respectively1, 2). Most ARF is reversible. The kidney is unique among the major organs in its ability to recover from almost complete loss of function. Nevertheless, ARF is associated with major in-hospital morbidity and mortality, in large part due to the serious nature of the illnesses that precipitate ARF3, 4).
Patients who are hypotensive and oliguric due to surgery, sepsis or bleeding are at risk of developing post-ischemic acute tubular necrosis (ATN). The two major histological changes in ATN are the loss of intact or necrotic tubular cells and occlusion of the tubular lumen by cellular debris; recovery of renal function after a period of one to three weeks in most cases is preceded by cellular regeneration by mitoses5, 6). Poor oxygenation leads to various changes, including the generation of reactive oxygen species, depletion of ATP and apoptosis. Apoptosis has been observed in the intact kidney subjected to transient ischemia7, 8).
Poor oxygenation results in either cell death or the sloughing of viable cells into the tubular lumen by impairment of normal cell-to-basement membrane adhesion9, 10). Ischemia-induced redistribution of membrane proteins can also directly interfere with tubular function. Sodium reabsorption normally occurs via the exit of sodium from the cells by the Na-K-ATPase pump in the basolateral membrane. Following ischemia in experimental animals, the actin cytoskeleton that anchors the Na-K-ATPase pump to the basolateral membrane becomes disrupted, allowing some of the pumps to redistribute onto the luminal membrane, thereby interfering with normal ion transport by pumping sodium back into the tubular lumen11, 12). Recovery of function is associated with recovery of normal polarity, including return of the Na-K-ATPase pump to the basolateral membrane11).
Previous studies have reported that markedly increased concentrations of a number of different classes of protein in cells are initially identified after various stresses, including heat shock13, 14). Heat shock protein (HSP) is a family of highly conserved proteins synthesized under various pathophysiological circumstances. These molecules are classified into five major subfamilies: HSP110, HSP90, HSP70, HSP60 and small HSP (sHSP). These chaperones are abundant and ubiquitous, and have constitutive functions in the synthesis and folding of proteins, translocation of proteins into membrane compartments, and the assembly and disassembly of oligomers15). HSP90, HSP70, HSP60 and sHSP are effective in maintaining unfolded proteins in soluble nonnative states conducive to refolding to the native state15).
In this study, the putative interactions between apoptosis and heat-shock proteins disturbed due to ATP depletion were investigated as a hypoxia model.
MATERIALS AND METHODS
The direct cellular damages were assessed by the release of LDH from the cytoplasm of human tubular epithelial cells (HK-2) after ATP depletion. The Bcl-2 /Bax mRNA expression ratio, used as an index to assess to what extent apoptosis contributed to tubular cell damage, and the expressions of HSP 90, 72 and 27 in relation to the Bcl-2/Bax ratio in the ischemic model as parameters of their functional contribution to tubule cell damage were also studied.
1. Cell and Chemicals
HK-2 cells were obtained from the American Type Culture Collection (CRL-2190: ATCC, Rockville, USA). HK-2 cells are proximal tubular cells derived from normal human kidney. They were immortalized by transduction with human papilloma virus 16 E6/E7 genes, which are known to retain functional characteristics of proximal tubular epithelium, such as Na+ dependent/phlorizin sensitive sugar transport and adenylate cyclase responsiveness to parathyroid, but not to anti-diuretic hormone. The cells were cultured in keratinocyte-serum free media (Gibco BRL, Grand Island, USA) and used when they reached 80% confluence. All chemicals and reagents were obtained from Sigma Chemical Co., St. Louis, USA.
2. Heat preconditioning
Flasks containing HK-2 cells for heat preconditioning were sealed and immersed in a 43°C temperature-regulated water bath for 1 h. Unheated control flasks were also sealed for 1 h, but the temperature maintained at 37°C. The flasks were then dried, unsealed, fed with fresh media and returned to 37°C for 8 hours.
3. Anoxic stress by adenosine triphosphate (ATP) depletion
The ATP content was reduced in cultured HK-2 cells by their exposure for 1.5 hours to glucose free DMEM (Gibco BRL, Grand Island, USA) containing cyanide (5 mM) and 2-deoxy-D-glucose (5 mM). This maneuver reduced the ATP content to less than 10% of the baseline value within 10 minutes and impaired cell survival16). They were then returned to keratinocyte serum free media (SFM) containing glucose (10 mM) and kept at 37°C in an incubator for 12 h to permit recovery from ATP depletion. Parallel media changes were made in the controls using glucose, but no cyanide, in DMEM and keratinocyte-serum free media containing glucose (10 mM), respectively.
4. Cell survival
The cytotoxicity induced due to anoxic stress was determined by the release of LDH into the media. To allow calculation of the total releasable LDH activity in each culture flask, the cells remaining 12h after anoxic stress were scraped off into HBSS/BSA and sonicated. The LDH activity (slope = deltaOD490/min) was measured in a colorimetric microplate assay based on the conversion of lactate to pyruvate, according to the method of Korzeniewski and Callewaert (LDH/LD kit, Sigma Chemical Co., St. Loius, USA)17). The cytotoxicity was expressed as a percentage of the total LDH activity released from the total LDH activity released into the cell conditioned media, according to the following formula, where MC and MU = the slopes of the conditioned and unconditioned media, respectively, and L=slope of lysate:
5. Total RNA Extraction and Reverse Transcription for Bcl-2 and Bax, HSP90, HSP72, HSP27 and GAPDH
Total RNA was isolated from HK-2 cells incubated with Keratinocyte-SFM alone or heat shocked at specific time intervals and from the control HK-2 cells before and 12 hours after ATP depletion. From the heat preconditioned HK-2 cells, total RNA was also isolated before and 12 hours after ATP depletion. An uneasy Mini Kit (Qiagen, Gmbk, Germany) was used for the isolation of total RNA. Complementary DNA was prepared with an RNA PCR Kit (Ver. 2.1, Cat. #R019, Takara Shuzo Co. Ltd., Otsu, Japan). Purified total RNA was dissolved in DEPC-DW (Diethyl Pyrocarbonate-Distilled Water: Sigma Chemical Co., USA), and 5 μg mixed with 2.5 μL of AMV reverse transcriptase XL (5 units/μL), 1.5 μL of RNase Inhibitor (40 units/μL), 2.5 μL of Oligo dT-Adaptor (2.5 pmol/μL), 5 μL of dNTP Mixture (10 mM each), 10 μL of MgCI2 (25 mM) and 5 μL of 10× RNA PCR Buffer in DEPC-DW to a total volume of 50 μL. The mixture was incubated at 42°C for 30min, 99°C for 5min and 5°C for 5min. The cDNA was stored at −20°C until required.
Bcl-2 and Bax were assessed with Apoprimer kits (Bcl-2 family: Takara Shuzo Co., Ltd., Japan). 50 μL PCR mixtures were prepared with 5 μL cDNA, 5 μL 10× PCR Buffer, 2 μL dNTP (2.5 mM each), 1 μL primer mixture (each 10 pmol/μL) and 0.5 μL Takara Taq (5 units/μL). Denaturation was conducted at 94°C for 30 sec, annealing at 59°C for 30 sec, extension at 72°C for 30 sec, with amplification performed for 35 cycles. The estimated product sizes of the Bcl-2 and Bax were 380 bp and 371 bp, respectively. Primer mixtures were used after heat treatment at 94°C for 2 minutes, with rapid cooling on ice to avoid dimer formation of the primers.
The primer sequences for HSP90, HSP72, HSP27 and GAPDH were designed with the help of the NCBI databank (http:/www.ncbi.nlm.nih, USA). The primer sequences used were as follows: Human HSP27, expected PCR product size of 225bp, sense 5′-GTC CCT GGA TGT CAA CCA CT-3′, anti-sense 5′-CTG AGC AAG GGA ATC AGG AG-3′; Human HSP72, expected PCR product size of 271bp, sense 5-GAG GTG GAG AGG ATG GTT CA-3′, anti-sense 5-GAC AGA TTT GCT CCA GCT CC-3′; Human HSP90, expected PCR product size of 412bp, sense 5-ATG CTC CAG CAG AGC AAA AT-3′, anti-sense 5′-TCC CAT CAA ATT CCT TGA GC-3′; Human GAPDH, expected PCR product size of 420bp, sense 5′-GTC AGT GGT GGA CCT GAC CT-3′, anti-sense 5′-AGG GGT CTA CAT GGC AAC TG-3′.
PCR was conducted with specific primer pairs. The final mixtures contained 5 μL of each cDNA, 5 μL of 10× PCR Buffer, 2 μL of dNTP 2 μL (2.5 mM each), 2 μL of sense primer (20 pmol/μL), 2 μL of anti-sense primer (20 pmol/μL) and 0.5 μL of Taq polymerase (5 units/μL), with DW to a total volume of 50 μL.
The PCR mixtures were placed in a Peltier Thermal Cycler (PTC-200: MJ Research, INC., Waltham, USA) and amplified 35 times.
The PCR products were subjected to electrophoresis on 1.7% agarose gels containing ethidium bromide (0.5 μg/mL for 20 min at 100 V. Photographs were taken under UV transillumination (Gel-Doc 1000, Bio-Rad, Hercules, USA). The approximate size was determined using a 100 bp DNA molecular weigh marker (Takara Shuzo Co. Ltd., Otsu, Japan).
6. Western blotting for HSP90, HSP72 and HSP27
When HK-2 cells seeded in 25 cm2 flasks reached 80% confluence, they were washed twice with ice cold PBS and trypsinized. The cell suspension was centrifuged for 5 min at 1,500 rpm and 4°C to obtain a cell pellet. The cell pellet was resuspended in 100 μL lysis buffer (50 mM Tris-HCI: pH 8.0, 5 mN EDTA: pH 8.0, 250 mM NaCl, 50 mM NaF, 0.1% Triton X-100, 50 ,μg Aportinin) on ice. The protein concentrations were determined by the method of Bradford, using bovine serum albumin as the standard. 100 μg of cytoplasmic protein were subjected to electrophoresis on 12% SDS-PAGE and transferred to a PVDF membrane (Amersham, Mississauga, Canada) in transfer buffer (25 mM Tris, 192 mM Glycine, 20% Methanol) for 4 hours at 100 V and 4°C. The membrane was blocked overnight at 4°C with phosphate buffered saline, containing 5% non-fat dried milk (w/v) and 0.1% Tween 20 (v/v), to avoid nonspecific reactions. HSP90, HSP70 and HSP90 were detected with monoclonal antibodies against HSP27 (Santa Cruz Biotechnology, Inc., Santa Cruz, USA), HSP70 and HSP27 (StressGen, Victoria, Canada) diluted 1:500, 1:1000 and 1:3000, respectively, with PBS containing 1% non-fat dried milk (w/v). An enhanced chemiluminescence system (Western blotting detection system, Amersham, Mississauga, Canada) was employed to detect each reactions.
7. Statistical analysis
All values are presented as the mean±SEM obtained from three separate experiments. Statistical analyses were performed using Wilcoxon signed rank sum tests (SPSS version 10.0, Chicago, IL). A 2-tailed p value<0.05 was considered significant.
RESULTS
1. Cell viability assessed by cytosolic LDH release
Cytosolic LDH release from HK-2 cells was determined before and after heat preconditioning. There was no difference in the cytolytic toxicity of the HK-2 cells subjected or not to the heat preconditioning. (5.67±1.15 vs. 5.33±1.15, p>0.05) (Table 1).
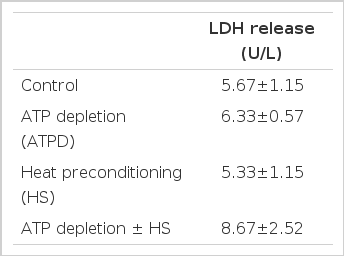
The changes of LDH with ATP depletion (ATP), prior to and after heat preconditioning (HS) versus the control
With ATP depletion, the LDH release from the HK-2 cells increased, and was slightly greater from the heat preconditioned cells However, the difference was not significant. (6.33±0.57 vs. 8.67±2.52, p>0.05) (Table 1).
2. Bcl-2 / Bax mRNA expression ratio
The Bcl-2/ Bax mRNA expression ratio increased progressively from the controls to the heat preconditioned to the ATP depleted cells (control; 100%, ATP depletion; 154±6%, heat preconditioning; 212±6%, heat preconditioning and ATP depletion; 421±8%) (Table 2).
3. The mRNA expressions of HSP90, HSP72 and HSP27
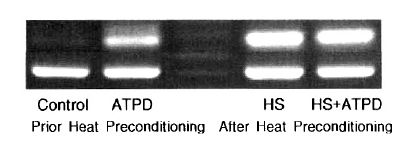
No contribution of heat preconditioning and ATP depletion was observed on the expression of HSP90 (Table 2). HSP72 expression was prominent with ATP depletion, but the expression of HSP72 was more evident after heat preconditioning (control; 100%, ATP depletion; 907±7%, heat preconditioning; 2556±7%, heat preconditioning and ATP depletion; 2398±8%) (Table 2, Figure 1). HSP27 expression was not evident after ATP depletion. The expression of HSP27 was increased slightly more after heat preconditioning (Table 2).
4. Western Blotting Analysis of HSP90, HSP72 and HSP27
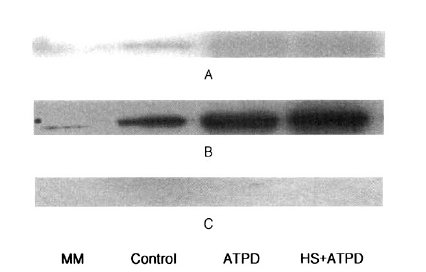
No contribution of heat preconditioning was observed on the expressions of HSP90 and HSP27 by Western blotting. HSP72 expression was prominent with ATP depletion, but that of HSP72 was more evident after heat preconditioning (Figure 2).
DISCUSSION
Although ischemia induced necrosis of renal epithelial cells is a well established pathological change, apoptosis is increasingly recognized as an important mechanism of cell death and organ dysfunction. The importance of apoptosis as a cause of death in ATP-depleted renal epithelial cells is also supported by the observation that two different caspase inhibitors are known to prevent the execution phase of apoptosis and to improve cell survival18).
This study employed cyanide treatment as a model of hypoxia. Early cellular functional changes of apoptotic injury were assessed by determining LDH release from the cells15). The anti-apoptotic Bcl-2 and pro-apoptotic Bax are known as important contributors in controlling the apoptotic process, and the ratio of Bcl-2 and Bax in renal cells plays a central role in regulating cellular sensitivity to numerous apoptotic stimuli19, 20). A reduction in Bcl-2, coupled with an increase in Bax, strongly favors apoptosis21). In our study, ATP depletion itself provided some increase in the Bcl-2/Bax mRNA ratio, but not as much as with heat preconditioning. Considering the non-significant LDH change after ATP depletion, the increase in the Bcl-2/Bax mRNA ratio would reflect the preservation of the cells from ATP depletion.
Several studies have shown that prior heat exposure and/or the selective accumulation of HSP inhibit apoptosis22–24). Heat stress proteins are induced by a variety of stimuli, including elevated temperature, ischemia, hypoxia, pressure overload and some chemicals. Heat, both quantitatively and qualitatively, is one of the best inducers of heat stress proteins23). In non-renal cells, HSP72 may alter several early events in the apoptotic signal cascade, including NF-kB, TNF, c-myc, and stress kinases, such as JUN-N-terminal kinase (JNK) and p3825). Wang et al. reported that prior heat stress significantly reduced the number of apoptotic cells, attenuated the pro-apoptotic changes in the Bcl-2 and Bax contents and improved cell survival23). Prior heat stress markedly increases the binding between HSP 72 and Bcl-2, suggestings a novel mechanism by which HSPs, such as HSP 72, attenuates renal epithelial cell death following an ischemic insult.
This study investigated the expressions of HSP90, HSP72 and HSP27 of the cells subjected to ATP depletion prior and after heat preconditioning. The expression of HSP90 was not impressive after ATP depletion, even after heat preconditioning. HSP90 makes a vital contribution to the maturation of signal transduction proteins, such as steroid hormone receptors or protein kinases26). After ischemia, HSP90 is known to be rapidly and transiently induced in the pars recta of the proximal tubule and in the thick ascending limb of the loop of Henle. HSP90 depletes Apaf-1 and inhibits cytochrome c-mediated activation of pro-apoptotic caspase-927). However, this study showed no significant up-regulation of HSP90 after ATP depletion, even after heat preconditioning.
The HSP70 family are also constitutively expressed and induced by stress. Early ischemia allowed HSP72 to localize in the apical domain of proximal tubular cells, where alterations of the cytoskeletal terminal web and brush border are most conspicuous, and Na-K-ATPase of the basolateral membrane appears in the apical domain26, 28). Recovery of function accompanies the re-establishment of the membrane-cytoskeletal complex and the recovery of normal polarity, including return of the Na-K-ATPase pumps to the basolateral membrane for normal ion transport12). HSP72 readily binds to denatured proteins in the absence of ATP and processes and releases the substrate, with hydrolysis of ATP. Given its localization within mitochondria and its role in preventing cytochrome c release, preservation of Bcl-2 by HSP72 could account for the protection of mitochondria ATP production after severe hyperthermia or ATP depletion in heat preconditioning renal epithelial cells. Mitochondrial protection may be central in cell survival as mitochondrial integrity is a critical determinant of whether cells ultimately undergo apoptosis23). This study showed that heat preconditioning significantly up-regulated the expression of HSP72. ATP depletion up-regulated the expression of HSP72, but not as much as with heat preconditioning. Therefore, it can be suggested that our findings of preservation of the cytolytic damage and increase in the Bcl-2/Bax mRNA ratio were closely related with the increase in the expression of HSP72 following ATP depletion. In contrast, there were no increases in the expression of HSP27 with ATP depletion or heat preconditioning.
In conclusion, there may be a possibility that the preservation of cytolytic damage and increase in the expression of the Bcl-2/Bax mRNA ratio is related to the increase in the expression of HSP72 following ATP depletion as a hypoxia model. However, to evaluate potential interactions between HSP72 and bcl-2/bax, the degree of coimmunoprecipitation of these proteins will need to be investigated.