Hypersensitivity to Mosquito Bites Associated with Natural Killer Cell–derived Large Granular Lymphocyte Lymphocytosis: A Case Report in Korea
Article information
Abstract
Hypersensitivity to mosquito bites (HMB) is characterized by intense skin reactions at bite sites. The pathogenesis of HMB might be related to clonal lymphoproliferation of Epstein-Barr virus DNA-positive natural killer (NK) cells. We report the first case of HMB possibly associated with NK cell-derived large granular lymphocyte (NK-LGL) lymphocytosis in Korea.
INTRODUCTION
Hypersensitivity to mosquito bites (HMB) is characterized by intense skin reactions at bite sites, which consist of not only erythema or bulla but also ulcer or scar, and is associated with general symptoms, such as high fever, general malaise, cramps and renal failure1). While this disorder is very rare and reports are scarce in the western literature, some cases of HMB have been reported from Japan2–4) and Taiwan5).
More than 90% of adults have been exposed to Epstein-Barr virus (EBV) and the virus persists in B cells and epithelial cells in the oropharynx. Recently, EBV has been considered to be associated with not only B-cell malignancy but also T-cell malignancy and natural killer (NK) cell granular lymphocyte proliferative disorder (GLPD)6). Here we report the first case of HMB associated with NK cell-derived large granular lymphocyte (NK-LGL) lymphocytosis in Korea.
CASE
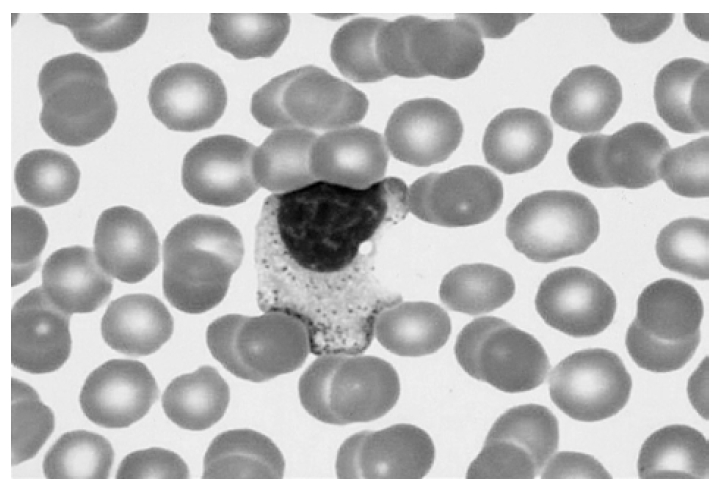
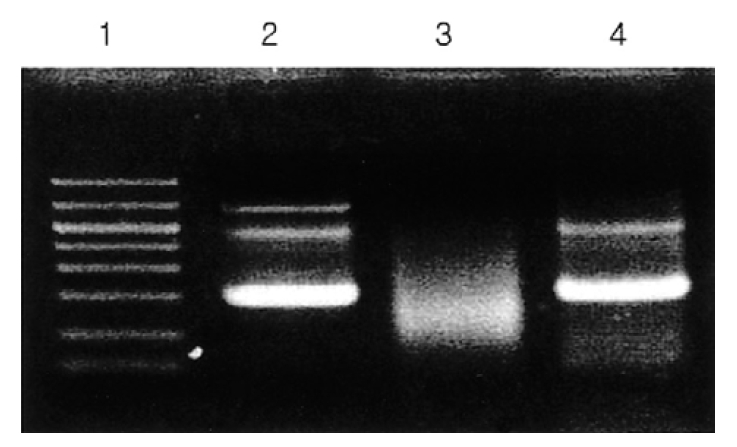
A 19-year-old male was referred to our hospital with well-demarcated pustules on the erythematous base on the face/right ear (Figure 1) and with fever for 4 days. He had suffered hypersensitive reactions to mosquito bites, such as skin lesions (ex, nodules, pustules, ulcerations), edematous change on the whole body and the extremities and fever, from childhood. Hepatosplenomegaly or peripheral lymphadenopathy was not detected. Laboratory tests showed white blood cell count 7.0×109/L, hemoglobin 15.0 g/dL, platelet 272×109/L, biochemical profile, including lactate dehydrogenase, was in the normal range. EBV anti-EA-DR IgG, anti-EBNA was positive and serum titer of IgG against EBV VCA increased. Peripheral blood smear revealed many large granular lymphocytes (Figure 2). Immunophenotypic analysis demonstrated that CD16+CD56+ cells increased (79%) and CD3+, CD4+, and CD8+ cells decreased (15%, 8%, 7%). We found rearrangement of TCRγ-chain gene by PCR analysis in this case (Figure 3), as in some cases of the GLPD7). In our case, Vγ-Jγ consensus primers (5′AGGGTTGTGTTGGAATCAGG3′ and 5′CGTCGACAACAA GTGTTGTTCCAC3′) for TCRγ-chain gene were used. PCR amplification with specific primers for TCRγ-chain gene demonstrated a single band of approximately 160–190 bp for Vγ-Jγ products. Bone marrow aspiration and biopsy revealed no abnormalities. Finally, we diagnosed this patient as NK-LGL lymphocytosis associated with HMB.
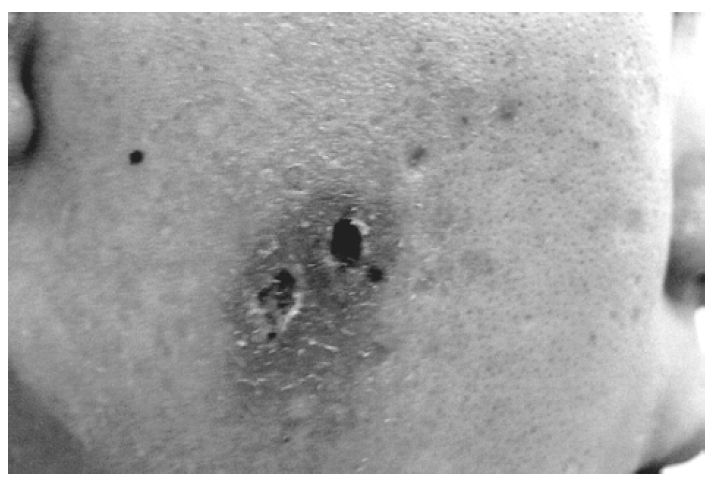
Skin lesions showed pustules, ulcerations, eschars and edematous change on the face and the right ear.
DISCUSSION
EBV, a widespread human herpes virus, infects >90% of the population by adulthood and persists in B cells and epithelial cells in the oropharynx where reactivation and viral replication may intermittently occur8). The virus has also been linked to various B cell, non-B cell neoplasms, such as endemic Burkitt’s lymphoma and nasopharyngeal carcinoma9). EBV can infect T cells and peripheral lymphoproliferation of CD3+ cells may occur under CAEBV and, also, EBV can infect NK cells and may induce NK cell lymphoproliferation in patients with CAEBV. If the activity of EBV is responsible for the lymphoproliferation, the anti-EBV antibody titers, especially anti-VCA IgG or anti-EA IgG might be related with lymphoproliferation. However, if our patient's data, such as EBV anti-VCA IgG and EBV anti-EA IgG, could not indicate CAEBV directly, this patient may be considered as CAEBV because NK cell lymphocytosis associated with EBV infection is frequently detected. To confirm the CAEBV, further examination of EBV, serial check-up for serum levels of anti-VCA IgG, IgA and IgM, anti-EA IgG, IgA and IgM, and anti-EBNA are required.
According to Ishihara et al, 31% of cases of CAEBV were complicated by HMB and suggested that the pathogenesis of HMB might be related to clonal lymphoproliferation of EBV DNA-positive NK cells4). This immunohematological abnormality may induce the characteristic symptoms of HMB. On the other hand, Ishihara et al suggested that HMB might be one of the factors that induce EBV-associated lymphoproliferative disease4). In this study, while three patients who did not manifest lymphoproliferation did not exhibit this history of HMB, four of six patients who did manifest monoclonal or oligoclonal lymphoproliferation had HMB.
Tokura et al have reported that NK cell-dominant mononuclear cells are infiltrated in mosquito bite sites of a severe HMB patient10). It could be speculated that accumulation of NK cells augments reactions on the skin and that these NK cells may directly or indirectly mediate the systemic symptoms by the mosquito bites. Recurrent and prolonged activated state of NK cells may induce additional genetic damage, including chromosomal changes that lead to the genesis of leukemias or lymphomas8).
In conclusion, we report the first case of HMB associated with NK-LGL lymphocytosis in Korea, and further investigation of HMB pathogenesis and the relationship between EBV-infected NK cells and subsequent oncogenesis of NK-LGL lymphoma, including chronic NK-LGL lymphocytosis, is needed.