Primary non-Hodgkin’s Lymphoma of the Bladder with Bone Marrow Involvement
Article information
Abstract
Involvement of the lower urinary tract by advanced non-Hodgkin’s lymphoma (NHL) has been reported in up to 13% of cases, but primary NHL of the urinary bladder is very rare. A 35-year-old man was admitted to our hospital with a chief complaint of gross hematuria with left flank pain on April 12, 2001. Cystoscopy revealed an edematous broad-based mass on the left lateral wall of the bladder, and transurethral biopsy showed NHL, diffuse large B-cell type. Abdomino-pelvic CT scan demonstrated left-side hydronephrosis and hydroureter with left proximal ureter infiltration and thickening of the left lateral wall of the bladder with perivesical fat infiltration without lymph node enlargement. Full-scale staging work-up revealed the bone marrow as the solely involved site. The lesions of the bladder and left urinary tract were nearly completely regressed after two cycles of systemic cyclophosphamide, doxorubicin, vincristine and predinisone (CHOP) chemotherapy with simultaneous restoration of urinary function.
INTRODUCTION
The usual presentation of non-Hodgkin’s lymphoma (NHL) is a localized or generalized lymphadenopathy, but in about one fourth to one third of cases it may be primary in other sites of the extranodal, where lymphoid tissue is found1). Among the common extranodal sites, head, neck, oropharyngeal region, gastrointestinal tract, bone marrow, skin and central nervous system are known to be commonly involved organs. Primary presentation of NHL in the bladder is exceedingly rare, although the lower urinary tract involvement occurs in up to 13% of patients with advanced disease arising from other sites2–7).
Hematuria is the most common initial presentation in bladder lymphoma and urinary frequency and dysuria are often accompanied4–6). The primary lymphoma in the urinary bladder has been reported to have a relatively benign clinical course, especially that with favorable histology7). On the other hand, there is no report on the clinical course of primary bladder lymphoma with aggressive histology and bone marrow involvement.
We herein report a very rare case of primary NHL of the urinary bladder with bone marrow involvement.
CASE
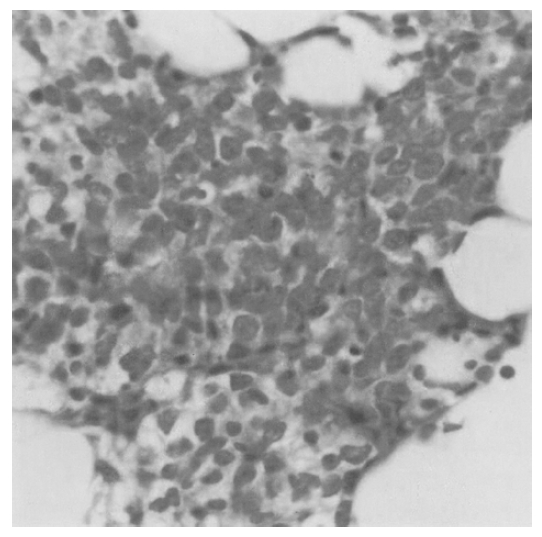
A 35-year-old man with painless gross hematuria with left flank pain of two-month duration was admitted to our hospital on April 12, 2001. He gave no history of B symptoms and did not have a significant medical history. There was no enlargement of the liver, spleen or lymph nodes. Evaluation at that time included normal findings on chest X-ray films, serum chemistry, complete blood count and serum protein electrophoresis, except slightly elevated levels of blood urea nitrogen, creatinine and increased lactate dehydrogenase. To evaluate gross hematuria, intravenous pyelography (IVP) and cystoscopy were performed. IVP showed a lobulated filling defect on the left wall of the urinary bladder associated with left hydronephrosis and hydroureter. Cystoscopy revealed an edematous walnut sized and broad-based mass on the left ureteral orifice and bladder neck. Histological examination of the biopsy taken transurethrally from two parts of the bladder mucosa and urethra showed malignant lymphoma (Figure 1A). Immunohistochemical study showed positive reaction for the monoclonal antibody marking B-lymphocytes (Figure 1B). For stage evaluation, chest CT, abdomino-pelvic CT and bone marrow biopsy were performed. Chest CT scan showed no visible lymph node or mass. Abdomino-pelvic CT scan demonstrated left side hydronephrois and hydroureter with left proximal ureter infiltration and thickening of the left lateral wall of the bladder with perivesical fat infiltration without lymph node enlargement (Figure 2A, 2B). Bone marrow aspiration and biopsy revealed normocellular marrow with diffuse infiltration of lymphoid cells (Figure 3). The β2-microglobin level was 3.4 mg/L.
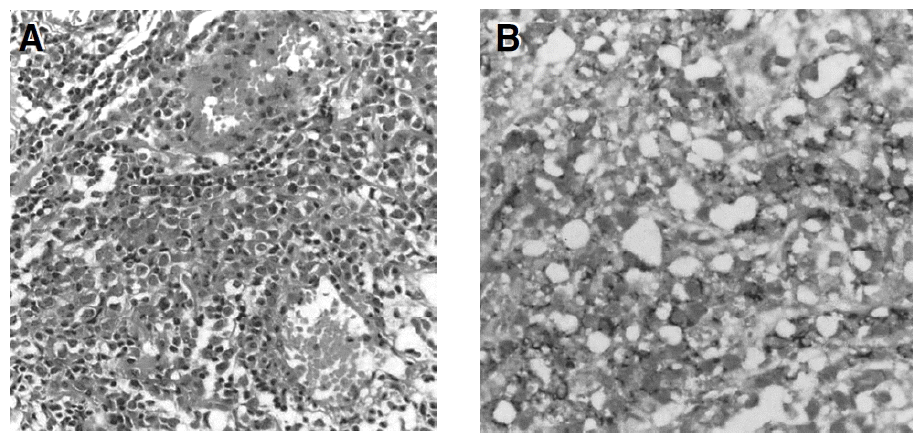
(A) Aggregation of monomorphous population of large lymphoid tumor cells (H&E, ×200). (B) Immumohistochemical staining shows a positive reaction for CD20, which indicates a lymphoma of B-cell origin (H&E, ×400).

Abdomino-pelvic CT scan demonstrates left-side hydronephrosis and hydroureter with left proximal ureter infiltration (A) and left lateral wall of bladder thickening with perivesical fat infiltration (B). CT scan after two cycles of CHOP chemotherapy shows the nearly complete regression of the previous lesions (C, D).
The final diagnosis was diffuse large B-cell lymphoma arising from the urinary bladder and ureter with bone marrow involvement. The disease was stage IV according to the Ann Arbor classification and intermediate histologic grade according to the Working Formulation. International Prognostic Index (IPI) score was 3 (high-intermediate group).
We started systemic chemotherapy with the cyclophosphamide, doxorubicin, vincristine and predinisone (CHOP) regimen (cyclophophamide 750 mg/m2, doxorubicin 50 mg/m2, vincristine 1.4 mg/m2 intravenously on day 1 and oral predinisone 100 mg/m2 on day 1 through 5). Eight cycles of CHOP were given every 3 weeks. The lesions of the bladder and left urinary tract were nearly completely regressed after two cycles of systemic CHOP chemotherapy with simultaneous restoration of urinary function (Figure 2C, 2D). After 18 months of follow-up, the patient is in complete remission (CR) status with normal bone marrow and no mass lesion is observed in the bladder.
DISCUSSION
Primary lymphoma of the bladder is reported to be very rare. It is known to have favorable prognosis if diagnosed early and treated appropriately2–8). The vast majority of bladder lymphomas are non-Hodgkin’s lymphoma of B-cell type and are much less frequently of diffuse large-cell type compared with other sites of extranodal lymphoma4,5,8).
Some authors reported that transurethral resection of the neoplasm, local aggressive surgical therapy or total nodal or total body radiation therapy, alone or in combination, produced long-term survival for patients with a localized disease with varying tumor histology5,9–11). On the other hand, some authors7) have claimed that localized primary lymphoma of the bladder, especially those with favorable histologic findings, often does not require medical or surgical intervention and persistent irritative bladder symptoms can be palliated adequately with external beam radiotherapy. Although the tumor is of low grade and confined to the bladder, systemic chemotherapy offers a good alternative and may be used, alone or in combination with radiotherapy8,12), but there is yet insufficient information on chemotherapy in malignant lymphoma of the bladder. Because most lymphomas are chemosensitive, however, treatment of micrometastasis is achieved by chemotherapy and radiotherapy may still be given to the pelvis at a later stage in the event of local recurrence. Usually, bladder lymphoma can be cured by aggressive local therapy, and the prognosis depends on the tumor stage and the specific lymphoma cell-type as defined by conventional histologic and immunologic criteria5).
In our case, the disease was stage IV according to the Ann Arbor staging system because lymphoma was not confined to the bladder but involved the bone marrow. According to our literature review, the present patient is the first case of stage IV bladder lymphoma. Also the case was of an intermediate grade disease by Working Formulation and B-cell neoplasm by WHO classification. It was classified as of high-intermediate risk with IPI score 3. The high-intermediate risk group comprises approximately 22% of NHLs with 55% of CR rate and 43% of 5-year survival rate13–15).
Conventional chemotherapy with the CHOP regimen or other equivalent third-generation regimens may be considered as the standard treatment for the good prognosis group16,17). However, for the poor prognosis group, the probability of long-term survival is less than 20%18,19). Because fewer than 50% of all patients are cured, it is essential to develop new and improved therapeutic approaches for patients with advanced-stage, aggressive-histology NHL. One phase II study of rituximab in combination with CHOP chemotherapy in patients with previously untreated, aggressive NHL demonstrated the safety and efficacy of this regimen, representing an overall response rate (ORR) of 100% and a CR rate of 67% in patients with IPI scores less than 2, and an ORR of 89% and CR rate of 56% in patients with an IPI score ≥220).
Involvement of the marrow was found to be dependent on both the histologic type and the extent of extramedullary disease21). Bone marrow involvement did not affect the survival of patients with low-grade NHL, but the survival was shorter for patients with intermediate-and high-grade NHL22). The IPI has a predictive value of overall survival and failure-free survival, while bone marrow involvement with a diffuse or interstitial pattern is associated with significantly poorer overall survival21,23,24).
We administered the eight cycles of systemic chemotherapy using the CHOP regimen based on the experience of others who have demonstrated better survival rates with systemic chemotherapy in this stage of neoplasm16). An additional reason for administering chemotherapy was the good general performance of the patient. Moreover, the lymphoma was extended to the ureter, causing hydronephrosis and hydroureter, and it was impossible to be covered by one radiation field. We had to consider the possible toxicity elicited by a relatively large radiation field. At the present, the lesions of the bladder and left urinary tract were nearly regressed after two cycles of chemotherapy and the patient is in CR status after 18 months of follow-up.
In summary, primary malignant lymphoma of the urinary bladder with bone marrow involvement is extremely rare. So, we report such a case that showed CR after CHOP chemotherapy based on the experience of other stage IV lymphoma showing better survival rates with systemic chemotherapy. The treatment outcome has been good, further evidencing the favorable prognosis of primary vesical NHL.