Adenocarcinoma of Unknown Primary Site
Article information
Abstract
Background :
Metastatic cancer of unknown primary site occupies 0.5–10% of all diagnosed cancer patients and includes various tumors with diverse responses to systemic chemotherapy. Adenocarcinoma of unknown primary site (ACUPS), the most common subtype, has no standard treatment, rarely responds to conventional treatment and has a poor survival rate.
Methods :
The retrospective study was performed to investigate the clinical characteristics and the treatment outcomes of ACUPS.
Results :
Eighty-one patients with ACUPS diagnosed at Samsung Medical Center from May 1995 to July 1999 were included. The median age was 58 years (range, 29–77). The common sites of metastases were the lymph node, liver, lung and bone in order. In 49 of 81 patients (60.5%), the dominant tumor location was below the diaphragm. The majority of patients (76 of 81) were initially treated with systemic chemotherapy including cisplatin. Responses were evaluable in 70 of 76. Eighteen of 70 patients (25.7%) responded to chemotherapy and complete remission was observed in 6 patients. The overall median survival of 81 patients was 5.6 months. The median survival of the responding patients was 18.3 months but the median survival of the nonresponding patients was 4.6 months (p<0.01). In univariate and multivariate analysis, age, performance status and response to initial chemotherapy were significant prognostic factors for overall survival.
Conclusion :
We observed poor response to the treatment and survival rate in ACUPS, but complete remission and long-term survival were observed in a small number of patients.
INTRODUCTION
Metastatic cancer of unknown primary site is not uncommon and ranks 8th and occupies 0.5–10% of all cancers diagnosed. It is a syndrome including various tumors rather than a single disease1, 2). Adenocarcinoma of unknown primary site (ACUPS) takes a large portion of it. Therefore, it represents a clinical entity with diagnostic and therapeutic problems3–5). We performed this study to investigate clinical characteristics and treatment outcomes of ACUPS with patients who were diagnosed as ACUPS at Samsung Medical Center.
PATIENTS AND METHODS
1. Patients
Eighty-one cases diagnosed as ACUPS at Samsung Medical Center from May 1995 to July 1999 were studied for a retrospective analysis. The diagnosis of ACUPS was established after histologic confirmation from a metastatic site revealed adenocarcinoma, and all relevant performed tests could not establish the site of the primary tumor. These tests included complete physical examination, hematologic work-ups including complete blood cell count, biochemical tests including liver function test, urinalysis, fecal occult blood test and chest X-ray.
2. Methods
All patients were inquired for clinical history with very careful questionnaires. Female patients underwent breast palpation and pelvic examination. Male patients underwent physical examination, including testis and prostate palpation. For definite pathological diagnosis, tissue biopsy or cytology of body fluid was examined. When it was required, immunoperoxidase stain of cytokeratin, neuron-specific enolase, β-human chorionic gonadotropin (β-hCG), S-100, prostate-specific antigen (PSA) and estrogen receptor was performed to get information about a possible primary site. If a possible primary site was suggested by the above tests, the computed tomography of chest or abdomen or pelvis, mammography, gastric endoscopy and tumor marker test were performed.
Initiation of treatment was dependent upon the age and performance status. We selected the chemotherapeutic regimen based upon clinical judgment considering the main site of involvement.
The survival analysis was assessed by the Kaplan-Meier limit-product method with log-rank comparison. Multivariate analysis was performed using the Cox regression hazard model for an independent prognostic factor.
RESULT
1. Clinical Characteristics
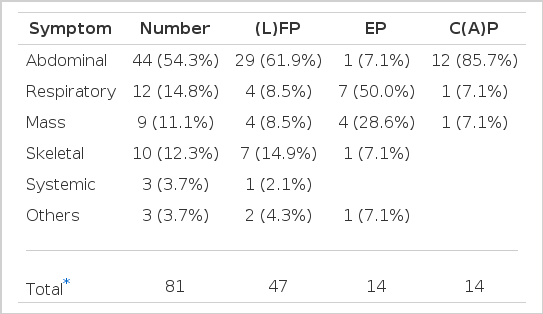
The ratio of male and female was 1:1.3. The median age was 58 years (range, 29–77). The performance status (ECOG) 0–1 was 67.9% (55 cases) and the tumor markers were abnormal in 77.8% (63 cases). CEA levels were high in 34 cases (42%). The number of metastatic sites was less than or equal to 2 in 51 cases (63%). In 49 of 81 patients (60.5%), the dominant tumor location was below the diaphragm. Ascites was observed in 25 cases (30.9%) and pleural effusion in 16 cases (19.8%) (Table 1). Apparent initial predominant symptoms were abdominal, respiratory, musculoskeletal symptoms and mass palpation in order (Table 2). Common metastatic sites were lymph node (42 cases), liver (16 cases), lung (13 cases) and skeletal system (12 cases). When the histopathologic finding was reviewed, the analysis of the grade of differentiation was possible in 53 cases. Among them, poor differentiation was observed in 29 cases (54.7%), moderate differentiation in 21 cases (37.6%) and well differentiation in 3 cases (5.7%).
2. Treatment Outcomes
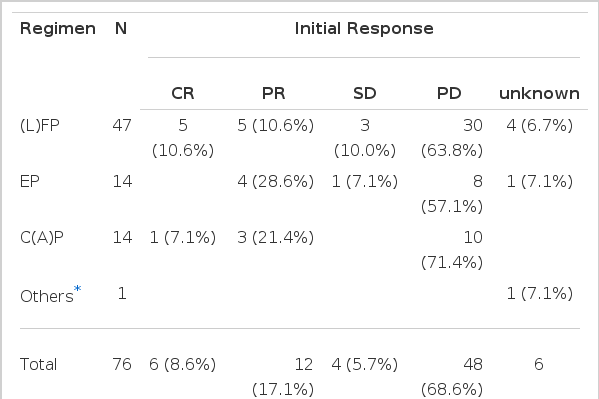
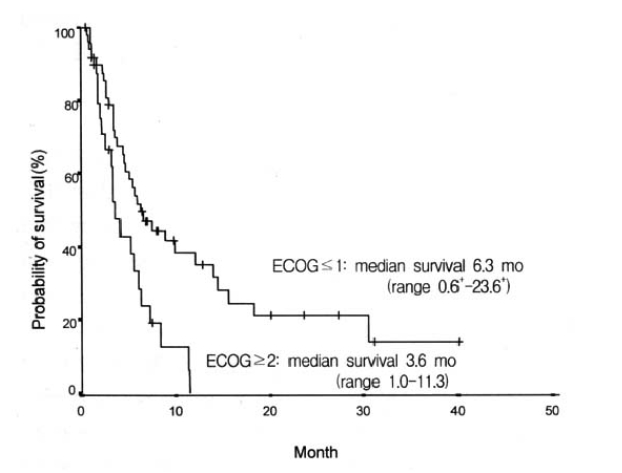
Considering the performance status, we treated 76 patients with systemic chemotherapy. Most cases used cisplatin-based chemotherapeutic regimen. Forty-seven patients received 5-fluorouracil (5-FU) and cisplatin as initial chemotherapy. Cyclophosphamide and cisplatin were administered in 14 cases, and VP-16 with cisplatin in 14 cases. The initial response to the systemic chemotherapy was observed in 18 cases (23.7%), including 6 complete response (7.9%). The median duration of response was 11.9 months (range, 1.63–34.2+). Sixty-nine percent (48 cases) of the total patients showed progression of the disease during the initial treatment. Table 3 shows the response to each chemotherapy regimen. The median survival of all patients was 5.6 months (range, 0.6–40.1+) (Figure 1), while the median survival of the patients with ascites or pleural effusion was 3.9 months and 8.9 months, respectively. The median survival of patients with the main site of involvement below vs above the diaphragm was 5.1 months and 12.1 months, respectively, which was not statistically significant (p>0.05). When the age group was divided by 60 years old, the median survival was 7.2 months in the age group less than 60 years, which was significantly longer than 3.6 months in the age group more than 60 years (p<0.05) (Figure 2). There was a significant difference between the two groups in survival by the initial treatment response. The median survival was 18.3 months in the responding patients and 4.6 months in the nonresponding patients (p<0.01) (Figure 3). The univariate analysis for age, performance status, tumor marker, initial predominant symptoms, metastatic sites, number of metastatic sites and systemic chemotherapy revealed that performance status was a significant prognostic factor (Table 4, Figure 4). The multivariate analysis showed performance status and response to initial treatment as significant prognostic factors (p<0.05).
DISCUSSION
ACUPS is an ill-defined syndrome which presents major diagnostic and therapeutic challenges. Despite extensive clinical investigation, a primary site of the disease is unlikely to be found; in approximately 15–27% of patients, a primary site cannot be found even at autopsy6, 7). It has a lot of clinical problems with poor prognosis as there is no standard treatment yet. There are many studies about ACUPS, but most of these studies emphasize metastatic cancer of unknown primary site9–12). There is no clear standard for testing to find a primary site when diagnosed as ACUPS. However, a minimum test based on clinical backgrounds such as clinical history and physical examination is recommended instead of aggressive tests8–11, 13). Most patients for this study underwent computed tomography in addition to basic laboratory tests. Tumor marker tests for CEA, CA-125, α-FP and β-hCG were performed considering the clinical status.
Various studies have been made about the treatment of ACUPS. In case of poorly differentiated ACUPS, it is known to respond well to chemotherapy with cisplatin and generally has a good response when the tumor is located in the mediastinum, retroperitoneum or lymph node. Greco et al.14) reported that there was a clinical improvement of more than 50% and a possibility of complete response in 10–15% of poorly differentiated ACUPS with cisplatin-based chemotherapy. Fiore et al.5) reported that treatment with vindesine and doxorubicin for patients with ACUPS displayed a median survival of more than 8 months in the responding group and 6 months in the nonresponding group. Sporn et al.15) recommended that such easily administered and less toxic regimen as 5-FU+Adriamycin+mitomycin was better for the treatment of ACUPS15). But it is absolutely clear that no chemotherapy can be a standard treatment for ACUPS. Unless a patient does belong to the group with expected high response after careful examination, the response to systemic chemotherapy is likely to be poor and one must make a prudent decision of treatment considering a possible toxicity related to the treatment. Also, any toxicity must be carefully monitored once chemotherapy is initiated and, if the response to treatment is not good, the treatment should be stopped.
A special subtype among ACUPS may show a good prognosis in which neuroendocrine differentiation, confirmed by electron microscope or immunoperoxidase stain displays a good response to systemic chemotherapy and a long-term survival can be expected16). Therefore, it is important for the decision of treatment policy and prognosis to perform a special staining and confirm the tissue subtype in patients with ACUPS. Meanwhile, it is reported that, contrary to other ACUPS, peritoneal carcinomatosis of unknown primary site in women shows a mild clinical course and a better response to chemotherapy with more possibilities of long-term and disease-free survival17).
Teo et al.18) investigated 42 patients with ACUPS and found that bone and lymph node metastases were common. Survival was short with a median duration of 4.6 months. It was also reported that a longer survival was observed in the responding group to chemotherapy than in the nonresponding group. We observed a lower response and survival rate to chemotherapy in this study, but it is consistent with the result of most other investigators. It is unlikely that substantial therapeutic progress will be made until better treatments are available for the underlying carcinomas that comprise this difficult clinical entity. However, in patients with good performance status diagnosed as ACUPS, an initial aggressive treatment should be considered to improve survival and to expect complete remission and long-term survival in a subset of patients.