Characterization of Binding and Phagocytosis of Oxidatively Damaged Erythrocyte to Macrophage
Article information
Abstract
Background:
Scavenger receptors are thought to be involved in the recognition of oxidized low-density lipoprotein (oxLDL) and oxidized erythrocyte (oxRBC). However, there are controversies about the kind of receptors and ligands related to the binding. Macrophages lacking class A scavenger receptor show identical binding of oxRBC with wild-type ones.
Methods:
RBCs were oxidized with ascorbic acid and CuSO4. Lipid oxidation was measured indirectly by measuring TBARS semiquantitatively. The binding and phagocytosis were measured by counting the number of oxRBC bound or taken up after incubation at 4°C or 37°C for 60 minutes to 100 macrophages differentiated from human monocytic leukemia cell line.
Results:
The degree of oxidation and the binding of oxRBCs were dependent on the concentration of CuSO4. The binding and phagocytosis of oxRBC were inhibited by 99% with oxLDL. Fucoidan, competing class A scavenger receptor, inhibited the binding by more than 90%. The binding of oxRBC was higher at 37°C than at 4°C by 3 times. The binding of oxRBCs was maximal at pH 6.5 and higher than at physiologic pH by 2.8 times. At pH 8.5 and 9.5, binding decreased by 67 and 88%, respectively.
Conclusion:
OxRBCs might bind and be taken up to macrophages not mainly through class A nor B scavenger receptors, but through other scavenger receptors and/or pathways. These processes are dynamic and ionic strength might be involved.
INTRODUCTION
Transformation of monocyte-derived macrophage to foam cell is one of the most important processes to develop atherosclerosis in the arterial wall1, 2). It is generally accepted that foam cell is formed by the uptake of oxidized low-density lipoprotein (oxLDL) through scavenger receptors1–5).
Although uptake of oxLDL by macrophage may have different qualitative and quantitative kinetics with that of plasma atherogenic lipoprotein, low-density lipoprotein (LDL), the same method is used to measure the amount of binding, association and degradation5–7). For LDL, the amount of degradation by the cell is higher by 30 times than that of the binding, mainly through a single receptor, LDL receptor6). For oxLDL, the amount of degradation is less than 10 times compared with that of binding through multiple receptors that have differences in the ligands' specificity5, 7–9). The ratio of degradation to binding of acetylated LDL (acLDL) is similar to that of LDL, although absolute amounts are higher by 20 fold in acLDL6–9). The difference might be due to the relatively higher binding capacity of oxLDL than that of LDL and due to the resistance of oxLDL to lysosomal degradation5, 8–10).
Red blood cell has been used to investigate the mechanisms by which damaged cells either aged, glycosylated or oxidated are removed11–13). Scavenger receptors are involved in the binding and uptake of oxidatively damaged red blood cell (oxRBC)13–17). OxRBC is recognized by a macrophage through a specific pathway for oxLDL. However, there are controversies about the kind of receptors and ligands related to the binding. Terpstra et al. reported that class A scavenger receptor was not related to the binding and uptake of oxRBC and that membrane phosphatidylserine mediated the recognition of oxRBC by scavenger receptor15). Beppu et al. reported that macrophage scavenger receptor recognized sialosaccharide chains of glycophorin of RBCs oxidized with diamide or periodate and that the binding was inhibited by ligands specific for scavenger receptor class A, such as fucoidan, dextran sulfate and polyinositol acid14).
Scavenger receptors have a broad range of ligand specificity3, 4). For scavenger receptor class A, electrostatic force is predominant in ligand-receptor interaction and this property might explain the diversity of ligands18, 19). There are no reports about the process involved in the binding of ligands to the scavenger receptor class B.
The present study was to characterize the processes for the binding and phagocytosis of oxRBCs to macrophage and to evaluate the usefulness of oxRBC as a tool for the kinetics of oxidized lipid based on several new findings observed.
METHODS
Materials
Phorbol-12-myristate-13-acetate, ascorbic acid, CuSO4, disodium-EDTA, trichloroacetic acid, thiobarbituric acid, NaOH, N-acetyl galactosamine and N-actyl glucosamine and fucoidan were purchased from Sigma. RPMI-1640, FBS, PBS and trypsin were purchased from Gibco. Ox-LDL was kindly provided by Dr YK Pak (National Institute of Health, Korea)20.
Cell culture
Human monocytic leukemia cell line (THP-1) was obtained from the American Tissue Culture Collection and was cultured in RPMI-1640 medium with 10% FBS at 37°C in 5% CO2. THP-1 cells were seeded in 12-well plate (5×105 cells/well) and differentiated into macrophage in the presence of 100 nM phorbol-12-myristate-13-acetate (PMA) for 72 hours21).
Oxidation of RBC
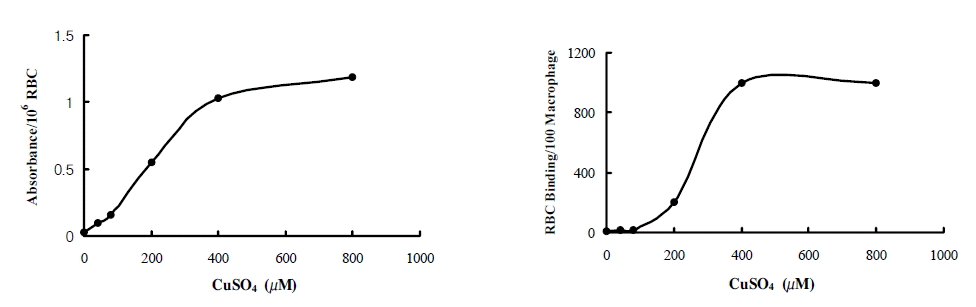
Blood was obtained in a tube containing disodium-EDTA from volunteers after overnight fasting. After centrifugation, plasma and buffy coat were removed. Remaining RBCs were washed three times with PBS and resuspended at 20% hematocrit in PBS. OxRBCs were prepared by the method described previously with minor modification13. Briefly, RBCs were incubated at 37°C for 90 minutes in the presence of 5 mM ascorbic acid plus CuSO4 ranging from 40 to 1600 μM (0, 40, 80, 200, 400, 800, 1200, 1600 μM) at 4% hematocrit in PBS. OxRBCs were washed once with PBS containing 2.5 mM EDTA and twice with PBS and resuspended in PBS at 2.5% hematocrit. RBC number, RBC volume and hemoglobin concentration were measured by automatic analyzer (Technicon H2, Bayer).
Measurement of oxidation
Lipid oxidation was measured indirectly by measuring TBARS semiquantitatively, as reported previously, with minor modification22). Briefly, 0.5 mL of 28% (W/V) trichloroacetic acid was added to 1 mL of RBC suspension in PBS at 2.5% hematocrit. After centrifugation, 0.8 mL of the supernatant was transferred to a new tube and 1 mL of 1% (w/v) thiobarbituric acid in 0.05 M NaOH was added. The mixture was boiled in the water bath for 15 minutes and cooled immediately under tap water. Absorption was measured at 532 nm using UV spectrophotometer (Ultraspec 2000, Pharmacia Biotech) and adjusted with the number of RBCs.
Assay for binding and phagocytosis
Macrophage differentiated from THP-1 cell was washed with PBS and oxRBC in PBS at 1% hematocrit was added. For binding assay, the plate was placed on the ice for 5 minutes before adding oxRBC. After incubation at 4°C or 37°C for 60 minutes, unbound oxRBCs were removed by washing twice with PBS. The number of oxRBC bound or uptaken to 100 macrophages was counted. Initially oxRBCs that were made at 8 different concentrations of CuSO4 from 0 to 1600 μM were used simultaneously. The number of bound oxRBC was counted and the number was considered as 1000 if binding was too many to count. To compare the binding and phagocytosis between the conditions, the concentration at which bound oxRBCs were between 50 and 500 in 100 macrophages was adopted. In a later experiment, only three concentrations of CuSO4 ranging from 200 to 600 μM were used. Figure 1 shows representative examples of the binding and phagocytosis of oxRBC to macrophage.

Representative examples of the binding (left) and phagocytosis (right) of oxidized RBC to macrophage
To investigate the influence of acidity on the binding, pH of PBS was adjusted from 5.5 to 9.5 using HCl or NaOH.
Statistical analysis
Data were expressed as mean±SE. For the comparison of RBC binding and phagocytosis, results were expressed as a percent of the control. Statistical analysis of the data was performed by Student t test. A value of p<0.05 was considered significant. The data presented were representative of at least 5 separate experiments.
RESULTS
Oxidation of RBC
The degree of RBC oxidation measured by TBARS semiquantitatively was dependent on the concentration of CuSO4 as expected (Figure 2, upper). There was a wide range of individual variation on the susceptibility of RBC to the extent of oxidation. In some cases, RBCs were fully oxidized at the concentration of 200 μM and in others at 1200 μM of CuSO4. Therefore, in each experiment we used oxRBCs that were oxidized at the different concentration of CuSO4. OxRBCs were very fragile and destroyed partially.
Effect of oxidation on the binding
The binding of oxRBCs was dependent on the degree of RBC oxidation (Figure 2, lower). There was no binding of native RBCs. With mild oxidation with low concentration of CuSO4, binding of RBCs was negligible. With extensive oxidation, bound oxRBCs were too many to count. The binding and phagocytosis of oxRBCs were compared at the concentrations at which the absorption curve was steep.
Inhibition of oxRBC binding by competing ligands
The binding and phagocytosis of oxRBC were inhibited by 99% with 100 μg/mL of oxLDL at all concentrations of CuSO4. Fucoidan at the concentration of 100 μg/mL also inhibited the binding by more than 90% at both 4°C and 37°C. N-acetyl galactosamine and N-acetyl glucosamine at 50 mM had no effect on the binding of oxRBC (Figure 3).
Effect of pH and temperature on the binding
The binding of oxRBCs was maximal at pH 6.5 and higher than physiologic pH by 2.8 times (Figure 4). At pH 8.5 and 9.5, binding decreased by 67 and 88% respectively, compared with that at pH 7.4. The binding of oxRBC was higher at 37°C than at 4°C (Figure 5) and the difference was influenced by the degree of oxidation. At the ideal zone for counting, binding of oxRBC was 3 times higher at 37°C than that at 4°C. At extensive oxidation, binding of RBCs was too many to compare.
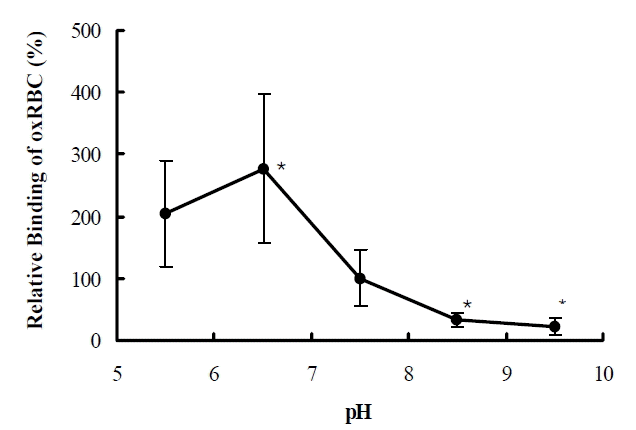
Effect of pH on the binding of oxidized RBC to macrophage. Relative percent binding of oxRBC to macrophage according to pH compared with that at pH 7.4. *p<0.05 compared with the binding at pH 7.4.
DISCUSSION
In this study, we characterized oxRBC-macrophage interactions by showing the relationships between the degree of oxidation of RBC and the binding and phagocytosis to the macrophage and new phenomena, such as the effect of pH and temperature.
Fatty streak is an early lesion of atherosclerosis and is mainly composed with foam cells that are derived from macrophages after uptake of modified LDL, maybe oxLDL1, 2). It is generally accepted that macrophage internalizes modified LDL primarily via a scavenger receptor, although other pathways, such as pinocytosis, may be involved23).
The method to measure the binding and uptake of oxLDL was adopted from that for LDL5–7). However, there are several different aspects between the two processes. LDL is bound and internalized to the cell mainly via a single receptor with limited ligands and oxLDL via multiple scavenger receptors with various ligands' specificities3, 4). For LDL, typical cell binding, association and degradation are 100, 600 and 3000 ng/mg protein respectively at the concentration of 10 g protein/mL for 5 hours6). For oxLDL, there are a few reports that presented quantitative measurement of cell binding, association and degradation simultaneously. The binding of oxLDL is higher by 7–12 times than that of LDL5, 6, 9).
In this experiment, we observed that oxRBCs bound to the culture plate nonspecifically and the more oxidation was associated with the more nonspecific binding (data not shown). Considering that oxLDL has the tendency to adhere nonspecifically, the higher binding capacity of oxLDL to the cells than LDL might be related to this phenomenon. In the experiment using oxRBCs, these nonspecific bindings can be excluded by direct visualization through microscope. This finding suggests that assay using oxRBCs be superior to that using oxLDL at least in several aspects for the assessment of the interaction between ox-lipid and macrophage.
OxLDL inhibited the binding and phagocytosis of oxRBCs completely. This finding is consistent with previous reports and indicates that oxRBCs bound to macrophage through all or part of the pathways of oxLDL binding10, 12). However, it is quite obscure which kinds of scavenger receptors mediate the process. Terpstra et al. reported that class A scavenger receptor was not involved in the binding of oxRBCs because macrophages lacking class A scavenger receptor showed same binding and uptake of oxRBCs17). Sambrano et al. reported that phosphatidylserine inhibited the binding of oxRBCs completely and that acLDL failed to compete15). These findings may mean that only class B or other scavenger receptors are involved in the binding of oxRBCs because phosphatidylserine binds to only class B scavenger receptors3, 4, 24, 25). However, because acLDL binds to both class A and B scavenger receptors with different affinities, no effect of acLDL cannot be explained based on known ligand specificity of scavenger receptors. In addition, Beppu et al. reported that antagonist of class A scavenger receptors, such as fucoidan or polyinosinic acid, inhibited the binding and uptake of oxRBCs although they used different methods for oxidation14). Sambrano et al. also reported that fucoidan or polyinosinic acid inhibited the binding and uptake of oxRBCs oxidized by CuSO413). In this study, fucoidan blocked the binding of oxRBC to macrophage completely and it suggests that class A scavenger receptor might be important in the binding of oxRBC. However, there is a possibility that other receptors or molecules that have not been fully identified yet and have overlapping ligand specificity may be associated to the binding of oxRBC, for example CD6815, 26, 27) or glycosaminoglycan28). Further study is needed for the identification of receptors or molecules related to the binding and phagocytosis of oxRBC.
Different results about the receptors related to oxRBC binding may be due to a difference in the macrophage used. Scavenger receptor expression is dependent on the cell type and activation. Resident macrophage has lower expression of scavenger receptor and activated macrophage by PMA or thioglycollate has higher expression. In addition, incubation time of macrophage might also make difference29).
There are controversies about the ligands related to the binding of oxRBC to macrophage. Beppu et al. suggested that scavenger receptor recognized oxRBC through sialosaccharide chains of glycophorin in resident peritoneal macrophage14). Several reports suggested that phosphatidylserine15), conjugation of lipid and protein11, 13, 30) or oxidized fatty acid8) on RBC membrane might be involved. There is a possibility that loss of sialic acid and exposure of galactosyl residue from glycoprotein or glycolipid on RBC surface by oxidation might be involved. It was suggested that old-aged erythrocyte might be removed from the circulation through this pathway11, 31). In this experiment, trypsin treatment to remove glycophorin from oxRBC and N-acetylgalatosamine, inhibitor for galactosyl residue, had no effect on the binding of oxRBC. These findings imply that the processes related to sialosaccharide chains of glycophorin or galactosyl residue are not involved in this experimental condition. The role of phosphatidylserine is also suspected because fucoidan, which does not bind to scavenger receptor class B32), blocks oxRBC binding completely and because phosphatidylserine binds to only class B scavenger receptor3, 4, 24, 25). There was a report that phophatidyhserine was not detectable on the outer membrane of diamide-treated erythrocyte33). It is possible that other receptors or molecules with similar but not the same property with phosphatidylserine mediate the process as described above. Further study is needed for the identification of molecules on oxRBC related to binding and phagocytosis of oxRBC to macrophage.
For the class A scavenger receptor, the receptor-ligand interaction appears predominantly electrostatic with specific spatial orientation of negative charge, base-quartet-stabilized four-strand helix18, 19). Class B scavenger receptors bind to a variety of lysine-modified proteins, anionic phospholipids, but not fucoidan and polyinositic acid3, 4). Electrical and structural requirements for ligands binding to class B scavenger receptor were not defined. In this study, as the binding of oxRBCs was dependent on pH, the involved process may be also mediated through electrostatic force. High affinity at acidic environment may have clinical implications and be helpful for macrophage to adhere to the inflammatory area where it is acidic due to poor perfusion and the accumulation of acidic molecules, such as lactic acid. High affinity at acidic environment might explain the low degradation rate and intracellular accumulation of oxLDL10, 34).
There were substantial differences of binding between at 4°C and 37°C, even though phagocytosis at 37°C was excluded. There are several possible explanations; further oxidation of oxRBC, appearance of new scavenger receptors, or involvement of other pathways with the exposure to oxidative molecules.
There are several limitations in this study. First, with the oxidation, RBCs were destroyed partially and the mean corpuscular volume measured by automatic analyzer decreased. So, total amounts of added RBCs were lower in wells with oxRBC. However, this limitation did not influence the results obtained using the same oxRBCs. Second, it was sometimes difficult to differentiate phagocytosis from binding. Therefore, there might be substantial personal variation in counting the number of phagocytosis. Third, the reproducibility of this method was limited. Intra-assay variation was 10%. Therefore, small differences cannot be discriminated with this method.
In spite of these limitations, we think that this method using oxRBC is very useful to investigate the interaction of receptor-ligand interaction of scavenger receptors with partial superiority to the classic methods using oxLDL. This method can control the nonspecific binding of ox-lipid that may be a confounding factor and is simple to handle. We showed several important aspects, such as pH and temperature dependency. We question the reason why there is a difference with the temperature and whether other mechanisms, in addition to scavenger receptor, are involved in the binding or not. Further studies are needed to answer these questions.
Notes
This study was supported by grant from Korean Circulation Society (2000-5).