A Case of Primary Adenosquamous Carcinoma of the Liver Presented with Liver Abscess
Article information
Abstract
Primary adenosquamous carcinoma of the liver is a very rare type of cholangiocarcinoma and is defined as a cancer containing both squamous and adenomatous components in the same lesion. Recently, we experienced a primary adenosquamous carcinoma of the liver presented as liver abscess. A 63-year-old man was presented with a 4-day history of fever and chill. The radiologic study showed a 4 cm-sized, central hypoattenuated mass with peripheral rim enhancement in the left lobe of the liver. Ultrasonography-guided aspiration and biopsy suggested an adenocarcinoma with abscess in the liver. At laparotomy, the tumor occupied the left lobe of the liver and invaded the right diaphragm. An extended left lobectomy and a partial excision of the involved diaphragm were done. Grossly, the tumor was 6×5×5 cm in size and had an eccentric necrosis. Microscopically, the tumor was composed of adenocarcinoma and squamous cell carcinoma with a transitional area.
INTRODUCTION
Primary adenosquamous carcinoma (ASC) of the liver is a rare subtype of cholangiocarcinoma. The incidence of ASC among cases of cholangiocarcinoma is 2–3%1). Although the clinicopathologic characteristics and biologic behavior of ASC of the liver have not yet been fully clarified, ASC tends to present more aggressive clinicopathologic features and has a worse prognosis than cholangiocarcinoma2, 3). The clinical symptoms are not characteristic. However, fever and abdominal pain induced by cholangitis were reported2, 3). ASC presented as liver abscess is extremely rare4). Recently, we experienced a rare case of primary ASC of the liver presented as liver abscess in a patient who had a previous history of liver abscess
CASE REPORT
A 63-year-old man was admitted to Korea University Ansan Hospital complaining of a 4-day history of fever and chill. He had a history of diabetes mellitus for 10 years and cholecystectomy due to gallstone 10 years ago. Four years ago, he was admitted to hospital due to liver abscess. At that time, he was treated medically and recovered without any complications. On admission, he was acutely ill-looking. The body temperature was 38.2°C. Abdominal examination revealed right upper quadrant tenderness without organomegaly. Initial laboratory evaluations were follows: WBC 10,000/mm3, Hb 11.0 g/dL, platelet 338,000/mm3, CRP 6.2 mg/dL, ESR 101 mm/hr, fasting blood glucose 236 mg/dL, AST 12 U/L, ALT 12 U/L, ALP 106 U/L, total bilirubin 0.1 mg/dL, total protein 6.9 g/dL and albumin 2.6 g/dL. HBsAg and anti-HCV Antibody were both negative. AFP, CEA and CA19-9 were 2.55 ng/mL, 20.8 ng/mL and 95.9 U/mL, respectively. Serum amoebic antibody was negative. An abdominal X-ray showed pneumobilia. Gastroduodenoscopy and endoscopic retrograde cholangiography demonstrated choledochoduodenal fistula. Colonoscopic findings were normal. Abdominal ultrasonagraphy revealed a complex echoic mass in the left lobe of the liver (Figure 1). Abdominal computed tomography (CT) showed a central hypoattenuated and peripheral rim enhanced mass in the left lobe of the liver without lymph node enlargement (Figure 2). These clinical and radiologic findings suggested a liver abscess. Under the impression of liver abscess, ultrasonography-guided fine needle aspiration was done. The cytological examination revealed some malignant cells and klebsiella pneumonia was cultured. For evaluation of the complex echoic mass of the liver, ultrasonography-guided percutaneous liver biopsy was performed. Histopathologic examination of the biopsy specimen revealed adenocarcinoma in the liver. At laparotomy, the tumor occupied the left lobe of the liver and invaded the right diaphragm. There was no lymphadenopathy in the adjacent area. An extended left lobectomy and a partial excision of the involved diaphragm were done. The specimen contained an ovoid yellow-gray tumor measuring 6×5×5 cm with an eccentric necrosis (Figure 3). The surrounding liver parenchyma showed several satellite tumor nodules. However, the specimen had free resection margin and any vascular invasion was not seen. Microscopically, the tumor was composed of two components: adenocarcinoma and squamous cell carcinoma components. The adenocarcinoma component was poorly differentiated whereas a few keratin pearls were observed in the squamous cell carcinoma component. The transitional area between adenocarcinoma and squamous cell carcinoma was recognized. Thus, a diagnosis of ASC in the liver could be made (Figure 4). The patient was transferred to the oncology department and received chemotherapy with 5-FU and cisplatin. He completed the 6th chemotherapy schedule and was well until 8 months after operation. The levels of CEA and CA 19-9 were within near normal range (5.3 ng/mL and 24.3 U/mL, respectively) and the follow-up abdominal CT did not show cancer recurrence.

Abdominal ultrasonography shows a complex echoic mass with peripheral rim in the left lobe of liver.
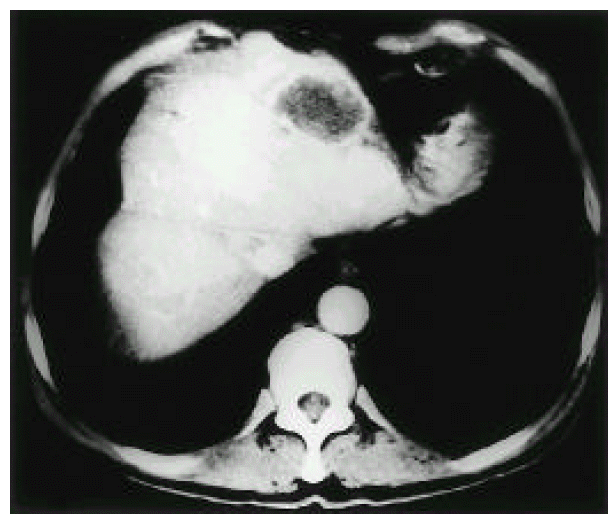
Enhanced abdominal computed tomography reveals a central hypoattenuated mass with peripheral rim-enhancement, measuring 4×3×3 cm in the left lobe of the liver.
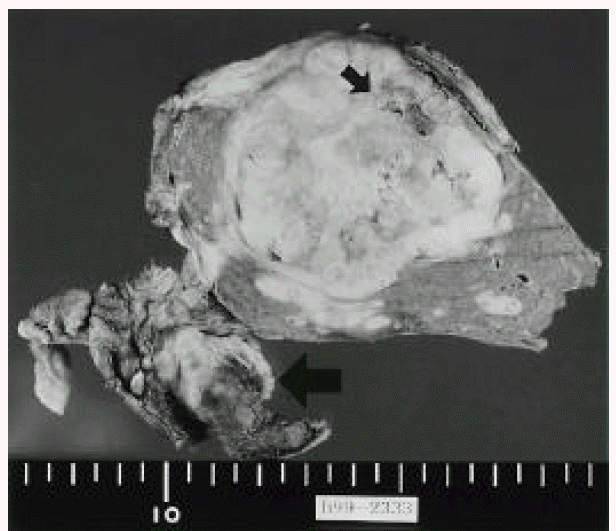
Resected specimen shows a solid and ovoid mass, measuring 6×5×5 cm with a necrotic area. Large arrow: falciform ligament, small arrow: necrotic area.
DISCUSSION
ASC sometimes develops in the pancreas5) and gallbladder5) but rarely in the liver. Carcinomas containing both adenocarcinoma and squamous cell carcinoma components have been referred to as ASC, adeno acanthoma and mucoepidermoid carcinoma. Since Pianzola and Durt7) described the first case of ASC of the liver in 1971, a few reports have described the clinicopathologic features of ASC2, 3, 8). ASC occurs between 40 and 85 years of age. The initial symptoms are commonly abdominal pain, fever, weight loss and jaundice. Preoperative diagnosis is extremely difficult. In our case, preoperative diagnosis was peripheral cholangiocarcinoma by percutaneous liver biopsy. ASC was confirmed postoperatively. The majority of ASC cases in the liver are detected at advanced stage. Most patients have a large mass and extensive invasion into the perihepatic region and lymphatics. In our case, there was an eccentric necrotic area that formed an abscess. The reported frequency of cholangiocarcinoma as the cause of liver abscess ranges from 2.4%–3.6%9). However, the incidence of liver abscess in ASC was not known. We found only one ASC with liver abscess in Medline search4). Therefore, abscess formation might be a very rare complication in ASC compared with common manifestations in cholangiocarcinoma.
Concerning the pathogenesis of ASC in the liver, several hypotheses have been proposed. Chronic inflammation of the bile duct, usually in association with infection and/or stones, has been proposed as an etiologic factor2). Such continuous irritations induce metaplastic changes in the biliary epithelium and lead to neoplasia3). In our case, persistent inflammation from the choledochoduodenal fistula may have played a role in the development of ASC. Other hypotheses3, 8, 10) suggested that ASC arose from squamous differentiation of adenocarcinoma cells. Recently, Maeda et al11) reported that most ASC of the liver develops from a squamous change of the cholangiocarcinoma, using the cytokeratins in the liver. In concordance with Maeda’s proposal, our case showed the transitional area that adenocarcinoma transit to squamous cell carcinoma. The adenocarcinoma component arising from the main biliary duct seemed to differentiate into the squamous cell carcinoma component centrifugally. The treatment of choice for this tumor is hepatectomy. Some report advocated radiation therapy, because squamous carcinoma is more sensitive to radiation therapy than adenocarcinoma3). However, the majority of patients died within 1 year after hepatectomy or diagnosis2, 11). In our case, the patient was treated with curative operation and post-operative adjuvant chemotherapy. Up to 8 months after operation, the patient was well and had no evidence of cancer recurrence.
